Standardization of the Hematopoietic Progenitor Assay Training Course
Two-day hands-on training on the CFU Assay for human cord blood and bone marrow samples
Sign Up
Please proceed to check out after completing this form. Payment information will be used to secure your registration and you will only be charged when the Intestinal Organoid Starter Kit is shipped.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
Overview
Speakers
Speakers will vary depending on course topic, date, and location.

STEMCELL Technologies

STEMCELL Technologies

STEMCELL Technologies
Agenda
Note that each day will end at approximately 4:30 pm and the course dinner taking place on one of the evenings.
Day One
-
Welcome Presentation and Introductions
-
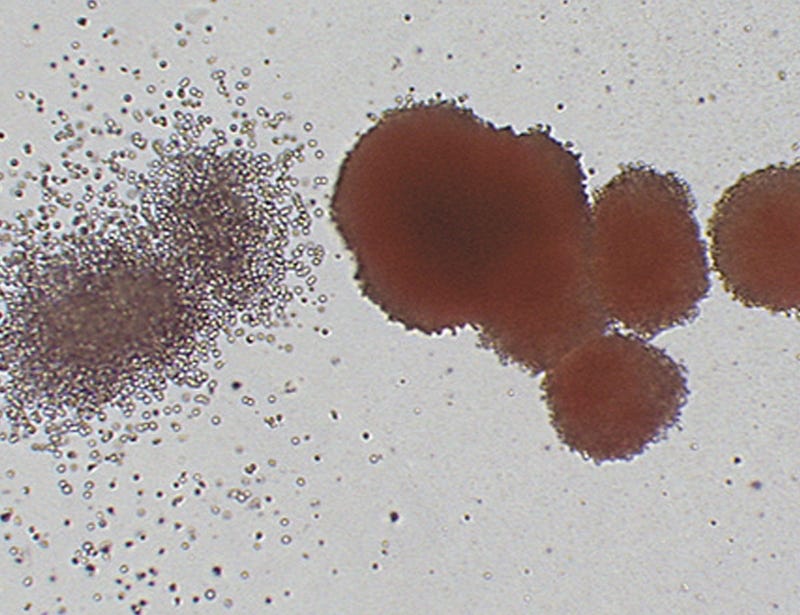
Lecture: Introduction to Hematopoiesis and the Colony-Forming Unit (CFU) Assay
-
Lecture 2: CFU Assay Set Up and Colony Identification
-
Practical Session 1 : CFU Assay Set Up Procedure
- Incubator maintenance
- Sample processing with ErythroClear™ Red Blood Cell Depletion Kit
- CFU assay setup
- Demo sample thawing and cell counting
-
Practical Session 2: Manual Colony Identification
-
Practical Session 3: Comparative Colony Counting
-
Wrap Up and Q&A
Day Two
-
Lecture: Standardization Tools for the Hematopoietic CFU Assay
-
Practical Session: Review of Manual Colony Identification Criteria and Comparative Counting
-
Practical Session: Review and Analysis of Group Comparative Counts
-
Lecture: Introduction to Automated and Standardized Colony Counting for the CFU Assay with STEMvision™
-
Practical Session: Operations of STEMvision™Wrap up Session:
- Q&A
- Quiz
- Course Evaluation
- Certificate Presentation
Accommodation
Vancouver, CA
Boston, US
Cambridge, UK (Transport included from this location to and from training venue - Cambridge UK only)
Dresden, DE
Contact education@stemcell.com for information on special rates (when applicable).
Policy
Travel Visas
Travel Visa Requirements
CanadaUnited States
United Kingdom
Germany
Cancellation
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (4)