EasySep™ Human Plasmacytoid DC Isolation Kit
Immunomagnetic negative selection kit
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
-
 EasySep™ Magnet
EasySep™ MagnetMagnet for column-free immunomagnetic separation
-
 EasySep™ Buffer
EasySep™ BufferCell separation buffer
-
Labeling Antibodies
Compatible antibodies for purity assessment of isolated cells
Overview
This product replaces the EasySep™ Human Plasmacytoid DC Enrichment Kit (Catalog #19062), for even faster cell isolations and is compatible with a greater number of EasySep™ magnets.
Data Figures
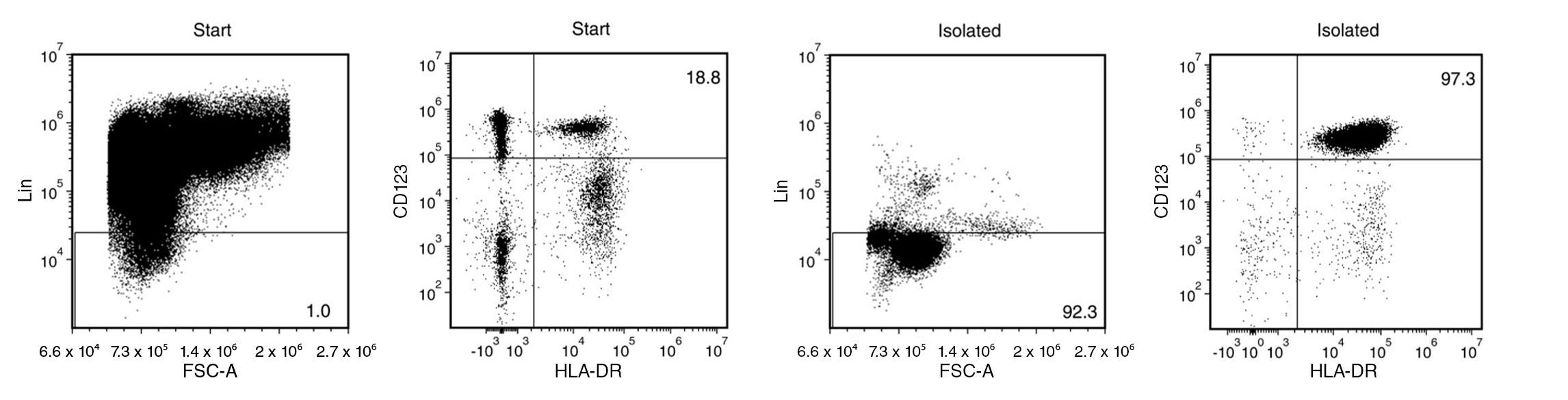
Figure 1. Typical EasySep™ Human Plasmacytoid DC Isolation Profile
Starting with leukapheresis samples, the pDC content (Lin-HLA-DR+CD123+) of the isolated fraction is typically 90 ± 5.3% (mean ± SD using the silver “Big Easy” EasySep™ Magnet). In the above example, the purities of the start and final isolated fractions are 0.2% and 89.8%, respectively.
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (5)
Related Products
-
 EasySep™ Human Pan-DC Pre-Enrichment Kit
EasySep™ Human Pan-DC Pre-Enrichment KitImmunomagnetic negative selection cell isolation kit
-
 Human Peripheral Blood Leukopak, Fresh
Human Peripheral Blood Leukopak, FreshPrimary human cells, fresh
-
 Human Whole Peripheral Blood
Human Whole Peripheral BloodPrimary human cells, fresh
Labeling Antibodies
-
 Anti-Human CD14 Antibody, Clone M5E2
Anti-Human CD14 Antibody, Clone M5E2Mouse monoclonal IgG2a antibody against human, rhesus, cynomolgus CD14
-
 Anti-Human CD20 Antibody, Clone 2H7
Anti-Human CD20 Antibody, Clone 2H7Mouse monoclonal IgG2b antibody against human, rhesus, cynomolgus CD20
-
 Anti-Human CD45 Antibody, Clone HI30
Anti-Human CD45 Antibody, Clone HI30Mouse monoclonal IgG1 antibody against human, chimpanzee CD45
-
 Anti-Human CD16 Antibody, Clone 3G8
Anti-Human CD16 Antibody, Clone 3G8Mouse monoclonal IgG1 antibody against human, rhesus, cynomolgus CD16
-
 Anti-Human CD123 (IL-3Rα) Antibody, Clone 6H6
Anti-Human CD123 (IL-3Rα) Antibody, Clone 6H6Mouse monoclonal IgG1 antibody against human, rhesus, sooty mangabey CD123 (IL-3Rα)
-
 Anti-Human HLA-DR Antibody, Clone LN3
Anti-Human HLA-DR Antibody, Clone LN3Mouse monoclonal IgG2b antibody against human, rhesus HLA-DR
Item added to your cart

EasySep™ Human Plasmacytoid DC Isolation Kit
Quality Statement:
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.