Reprogramming Human Urine-Derived Cells to Induced Pluripotent Stem Cells Using an Episomal Vector System in TeSR™-E7™ or ReproTeSR™
- Document # DX22917
- Version 1.0.1
- Nov 2023
The following protocol is for reprogramming urine-derived cells (UDCs) to induced pluripotent stem (iPS) cells using TeSR™-E7™ Medium for Reprogramming (Catalog #05914) or ReproTeSR™ Medium for Reprogramming (Catalog #05926), and an episomal vector system.
Equipment Required
Neon® Transfection System (Life Technologies Catalog #MPK5000)
Note: The method described here uses the Neon® Transfection System to transfect somatic cells with episomal vectors containing the reprogramming factors. Other vector systems or electroporation devices (e.g. Lonza Nucleofector™ or BioRad Gene Pulser Xcell™) may be used, but the protocol will need to be optimized for transfection efficiency and viability.
Materials and Reagents Required
Transfection
- Neon® Transfection System 100 μL Kit (Life Technologies Catalog #MPK10025)
Reprogramming Medium
Prepare either TeSR™-E7™ Medium for Reprogramming (Catalog #05914) or ReproTeSR™ Medium for Reprogramming (Catalog #05926). For complete instructions, as well as storage and stability, refer to the corresponding Product Information Sheet (PIS) for each product (Document #DX22190 or #DX22191, respectively), available at www.stemcell.com or contact us to request a copy.
Urine-Derived Cell Isolation and Expansion Media
- Prepare urine-derived cell isolation (UDCI) medium and urine-derived cell expansion (UDCE) medium by adding components as indicated in Table 1 to DMEM/F-12 with 15 mM HEPES (Catalog #36254). Adjust the volume of basal medium according to the calculated volumes of the additional components.
- Mix thoroughly.
NOTE: If not used immediately, store UDCI or UDCE medium at 2 - 8°C for up to 2 weeks.
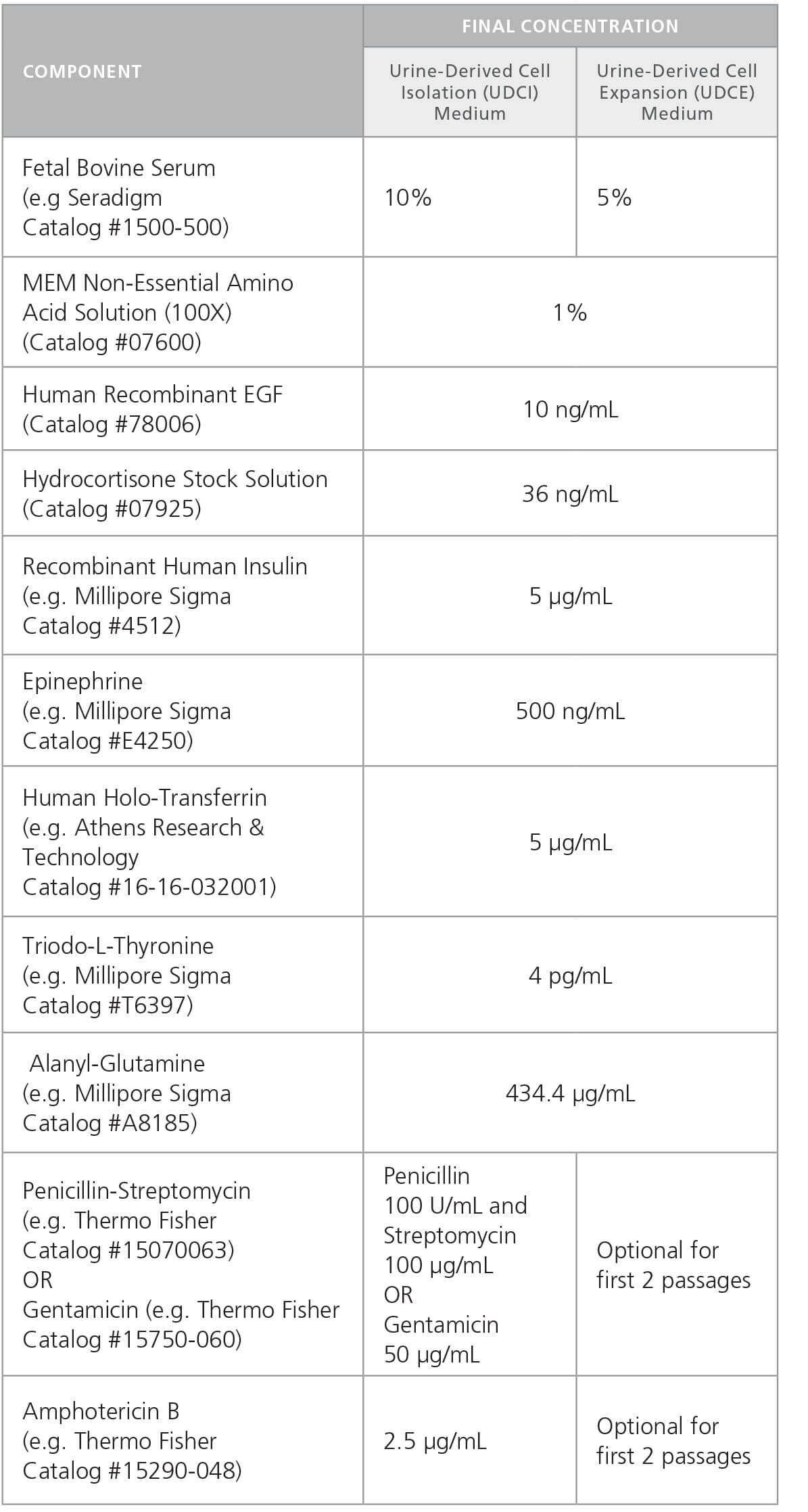
Table 1. Urine-Derived Cell Media Components and Recommended Stock and Final Concentrations
Vectors Encoding Reprogramming Factors
Episomal vectors available from Addgene:
- pCE-hOCT3/4 (Plasmid #41813)
- pCE-hSK (Plasmid #41814)
- pCE-hUL (Plasmid #41855)
- pCE-mp53DD (Plasmid #41856)
- pCXB-EBNA1 (Plasmid #41857) OR
- Epi5™ Episomal iPSC Reprogramming Kit (Thermo Fisher Catalog #A15960)
Other Materials and Reagents Required
Additional Supplementary Materials
Directions
Use sterile techniques when preparing reagents and performing the
following protocols.
The following protocols describe how to isolate and expand cells
from urine that are suitable for reprogramming. The number of
cells and growth characteristics of cells vary by donor (varying
age, sex, health status, etc.). On average, 100 mL samples of
urine contain sufficient cells to generate a culture suitable for
reprogramming. Success in establishing a culture improves with
increasing urine sample volume. Initial culture samples (particularly
female samples) may contain non-adherent cells that will be
removed during medium changes. The instructions below describe
an optimized procedure for use with 12-well tissue culture plates.
I. Derivation and Culture of Urine-Derived Cells
A. Isolation of Urine-Derived Cells
Process urine sample within 4 hours of collection. Success rate will decrease with delayed sample processing.
- Prepare sufficient UDCI medium, aliquot, and warm to room temperature (15 - 25°C). Add Y-27632 to a final concentration of 10 μM.
- Prepare a gelatin-coated 12-well plate by adding 0.5 mL of 0.1% Gelatin in Water per well and incubate at room temperature for at least 10 minutes.
- Transfer urine into sterile 50 mL conical tubes and centrifuge
at 300 x g for 5 minutes.
NOTE: If not used immediately, cryopreserve cells as described in section C. - Carefully remove supernatant, leaving a small amount (~1 mL)
in the tube. Gently pipette up and down to resuspend cells
and pool contents from all tubes into a single 50 mL conical
tube.
NOTE: Washing tubes with D-PBS and then transferring the wash to the tube containing the pooled sample may increase cell yield. - Add 10 mL of D-PBS to pooled sample and centrifuge at 300 x g for 5 minutes.
- Remove supernatant and resuspend cells in 1 mL of UDCI medium containing 10 μM Y-27632 (prepared in step 1).
- Remove excess 0.1% Gelatin in Water solution from the plate prepared in step 2. Transfer entire cell suspension to a single well of the gelatin-coated 12-well plate. Incubate at 37°C for 24 hours.
- Aspirate medium and add 2 mL of warm (37°C) UDCI medium.
- Perform full medium changes every 2 - 3 days with UDCI medium. Typically, colonies of cells appear around day 10, however this is subject to donor and sampling variability. Refer to Figures 1A - B for images of establishing UDC cultures.
- When the establishing passage 0 UDC culture becomes confluent (i.e the UDC colonies reach at least 1000 μm in diameter or the culture becomes > 30% confluent; refer to Figures 1A - B), cells are ready for passaging. Passage cells at a 1:1 split ratio for the initial passage using UDCE medium as described in section B steps 1 - 8.
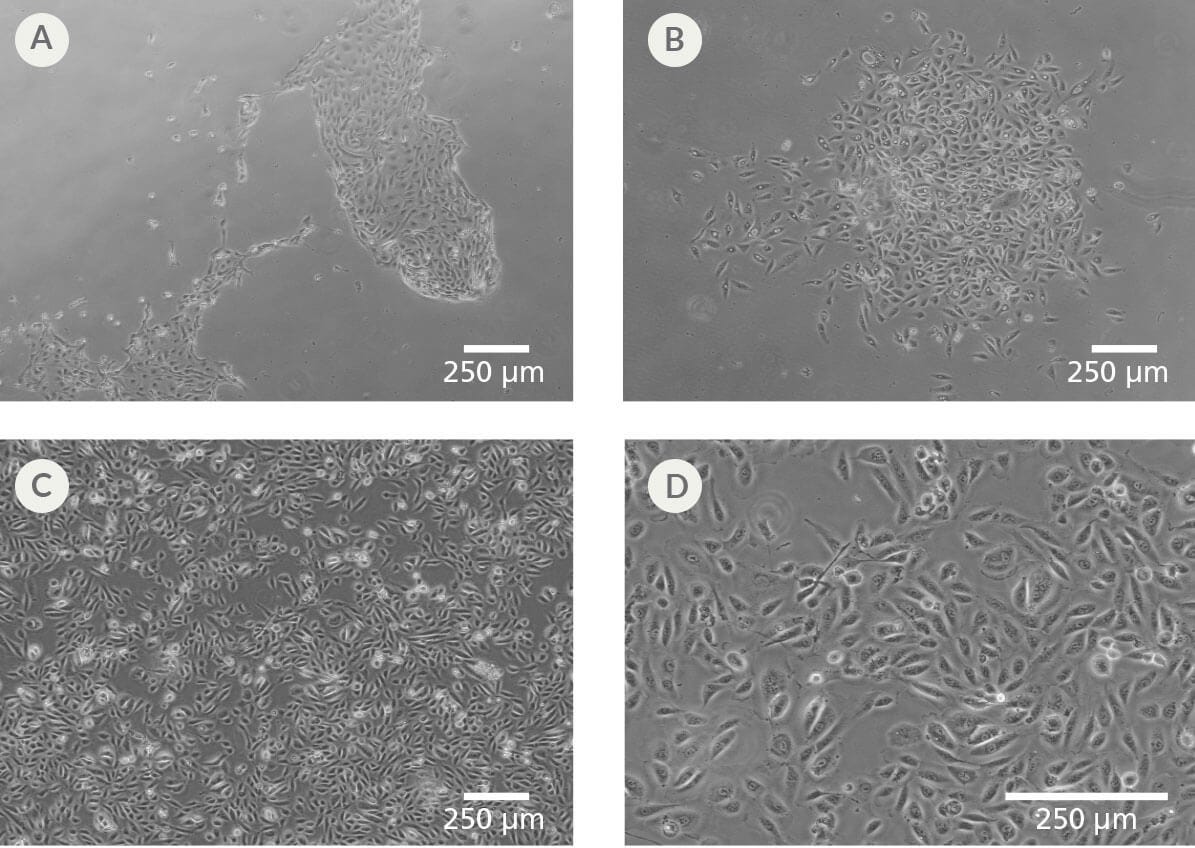
Figure 1. Human Urine-Derived Cells (UDCs)
(A-B) Representative images of establishing passage 0 UDC cultures grown in UDCI medium supplemented with amphotericin B and gentamicin for 9 days on 0.1% gelatin. (C-D) Representative images of established passage 1 UDC cultures grown for 3 days in UDCE medium on 0.1% gelatin.
B. Passaging Urine-Derived Cells
Starting with passage 1 when the UDC culture becomes 80 - 90% confluent (as shown in Figure 1C - D), cells are ready for passaging.
- Prepare sufficient UDCE medium and warm to room temperature (15 - 25°C).
- Prepare a gelatin-coated 12-well plate by adding 0.5 mL of 0.1% Gelatin in Water per well and incubate at room temperature for at least 10 minutes.
- Remove medium from cells and wash with D-PBS. Remove D-PBS.
- Add 500 μL Trypsin-EDTA (0.25%) per well. Incubate at room temperature for 3 - 8 minutes or until cells detach from the plate.
- Add 1 mL UDCE medium per well. Scrape cells off using a cell scraper and transfer cell suspension to a 15 mL conical tube.
- Centrifuge at 300 x g for 5 minutes.
- Remove supernatant and add UDCE medium to the cells. Perform a viable cell count using Trypan Blue and a hemocytometer, or an alternative cell counting method.
- Add 10,000 - 15,000 cells/cm2 to gelatin-coated 12-well plates. Incubate at 37°C.
- Perform full medium changes with UDCE medium every other day until sufficient cells are obtained for reprogramming. Monitor cultures, and if no contamination is detected, antimicrobial agents can be withdrawn from UDCE medium.
- Proceed to section II for reprogramming.
NOTE: Reprogramming efficiency of UDCs decreases with every passage; we recommend reprogramming UDCs at passage 4 or lower.
C. Cryopreservation of Urine-Derived Cells
When the UDC culture becomes 80 - 90% confluent (as shown in Figure 1C - D), cells are ready for cryopreservation, if desired.
- Remove culture medium and wash cells with D-PBS. Remove D-PBS.
- Add 500 μL Trypsin-EDTA per well and incubate at room temperature for 3 - 8 minutes, or until cells detach.
- Add 1 mL UDCE medium per well. Scrape cells off using a cell scraper and transfer cell suspension to a 15 mL conical tube.
- Centrifuge at 300 x g for 5 minutes.
- Remove supernatant, add cold (2 - 8°C) CryoStor® CS10, mix thoroughly, and transfer the cell suspension to a cryovial. For complete handling instructions refer to PIS (Document #29941).
- Freeze cells using a standard slow rate-controlled cooling
protocol (approximately -1°C/minute) or an isopropanol freezing
container and store at liquid nitrogen temperature (-135°C).
NOTE: Long-term storage at -80°C is not recommended.
II. Reprogramming Urine-Derived Cells
The following protocol (Figure 2) has been optimized for a 6-well plate. If using other cultureware, adjust accordingly.
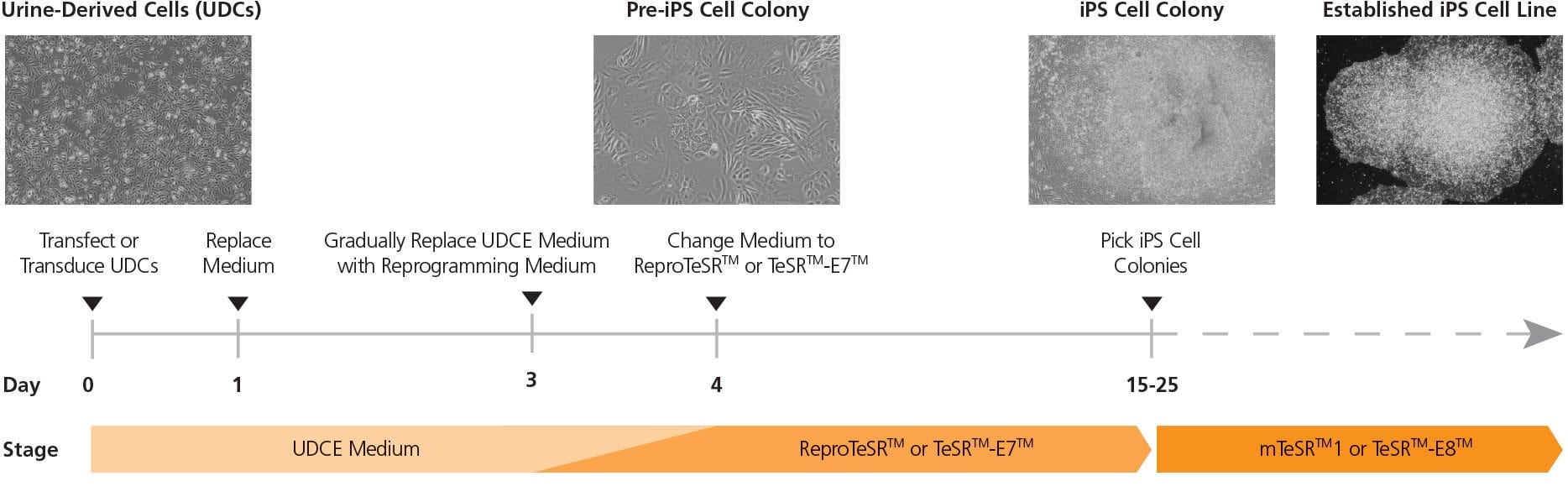
Figure 2. Reprogramming Timeline of Urine-Derived Cells Using an Episomal Vector System
UDCs are transfected with episomes on day 0, and cultured in UDCE medium. At 24 hours post-transfection, medium is replaced with fresh UDCE medium. After 3 days posttransfection, medium is changed to a 1:1 mix of reprogramming medium (ReproTeSR™ or TeSR™-E7™) and UDCE medium. Starting on day 4 post-transfection, cells are cultured in reprogramming medium (ReproTeSR™ or TeSR™-E7™) for the remainder of the reprogramming induction phase until iPS cell colonies emerge. By day 20, iPS cell colonies are typically large enough to be isolated and propagated in mTeSR™1 or TeSR™-E8™ media.
A. Coating Cultureware
Successful reprogramming of UDCs in TeSR™-E7™ or ReproTeSR™ requires that cultureware is coated with Corning® Matrigel® hESCQualified Matrix. For complete instructions on coating cultureware, refer to the Technical Manual for mTeSR™1 (Document #28315) or TeSR™-E8™ (Document #DX20809), available at www.stemcell.com or contact us to request a copy.
B. Preparation and Transfection of Urine- Derived Cells (Day 0)
- Prepare sufficient UDCE medium supplemented with Y-27632 to a final concentration of 10 μM for 2 mL/well plus an additional 10 mL. Add 1 mL/well to Matrigel®-coated plates.
- Aliquot 10 mL of UDCE medium supplemented with 10 μM Y-27632. Incubate the plates and the medium at 37°C until required in step 14.
- Prepare sufficient UDCE medium supplemented with Y-27632 to a final concentration of 10 μM for 2 mL/well plus an additional 10 mL. Add 1 mL/well to Matrigel®-coated plates.
- Aliquot 10 mL of UDCE medium supplemented with 10 μM Y-27632. Incubate the plates and the medium at 37°C until required in step 14.
- To prepare UDCs for electroporation, remove medium from cells and wash cells twice with D-PBS. Remove D-PBS.
- Add 1 mL Trypsin-EDTA (0.25%) per well of a 6-well plate. Incubate at room temperature for 3 - 8 minutes, or until cells detach.
- When the cells are fully detached from the flask, add 1.5 mL of UDCE medium. Transfer cell suspension to a 15 mL conical tube.
- Centrifuge at 300 x g for 5 minutes. Remove supernatant and resuspend cells in 5 mL D-PBS.
- Count viable cells using Trypan Blue and a hemocytometer, or an alternative cell counting method.
- Aliquot the appropriate volume of cell suspension into a conical tube to obtain sufficient cells for electroporation. We recommend starting with at least 1.25X the required amount of cells per reaction (6.875 x 105 cells, if setting up 1 reaction using 100 μL Neon® pipette tip). Centrifuge at 300 x g for 5 minutes.
- Remove supernatant and resuspend cells in the appropriate
electroporation suspension buffer to achieve a final
concentration of 5.5 x 106 cells/mL
Example: If using the Neon® Transfection System, resuspend 6.875 x 105 cells in 125 μL Resuspension Buffer R. Preparing 25% extra volume of cell suspension is recommended to avoid air uptake into the Neon® pipette tip. - Transfer cell suspension to a microcentrifuge tube. Add 4 μg of each episomal vector per million cells to the cell suspension and mix. For example, add 2.75 μg of each vector if the solution contains 6.875 x 105 cells.
- Electroporate cells with reprogramming vectors according to
the manufacturer’s instructions.
NOTE: If using the Neon® Transfection System, we recommend using 1200 V, 30 ms pulse width, 1 pulse in a Neon® 100 μL pipette tip. - Transfer transfected cells to a 15 mL conical tube containing 10 mL of warm UDCE medium supplemented with 10 μM Y-27632 (prepared in step 2).
- Seed 25,000 - 50,000 cells/well onto pre-warmed Matrigel®-
coated wells of a 6-well plate (prepared in step 2).
NOTE: Plating density may need to be optimized depending on growth kinetics of cells being reprogrammed. - Add sufficient warm UDCE medium supplemented with 10 μM Y-27632 to each well to a final volume of 2 mL/well.
- Incubate at 37°C for 24 hours. Proceed to section C for reprogramming induction phase.
C. Reprogramming Induction Phase in TeSR™-E7™ or ReproTeSR™
- Day 1: Aspirate medium from cells and add 2 mL of warm (37°C) UDCE medium per well. Incubate at 37°C for 2 days.
- Day 3: Aspirate medium and add 2 mL of a 1:1 mixture of reprogramming medium (TeSR™-E7™ or ReproTeSR™) and UDCE medium per well.
- Day 4 - 25: Aspirate medium and add 2 mL/well of reprogramming medium daily or every other day. During the initial period of reprogramming when the cell density is low (Figure 3A - B), medium can be changed every other day. Once colonies arise and medium begins to turn yellow, exchange the medium daily (Figure 3C - F).
- Proceed to section D for measuring reprogramming efficiency, and sections E - F for isolating colonies and culturing in maintenance medium.
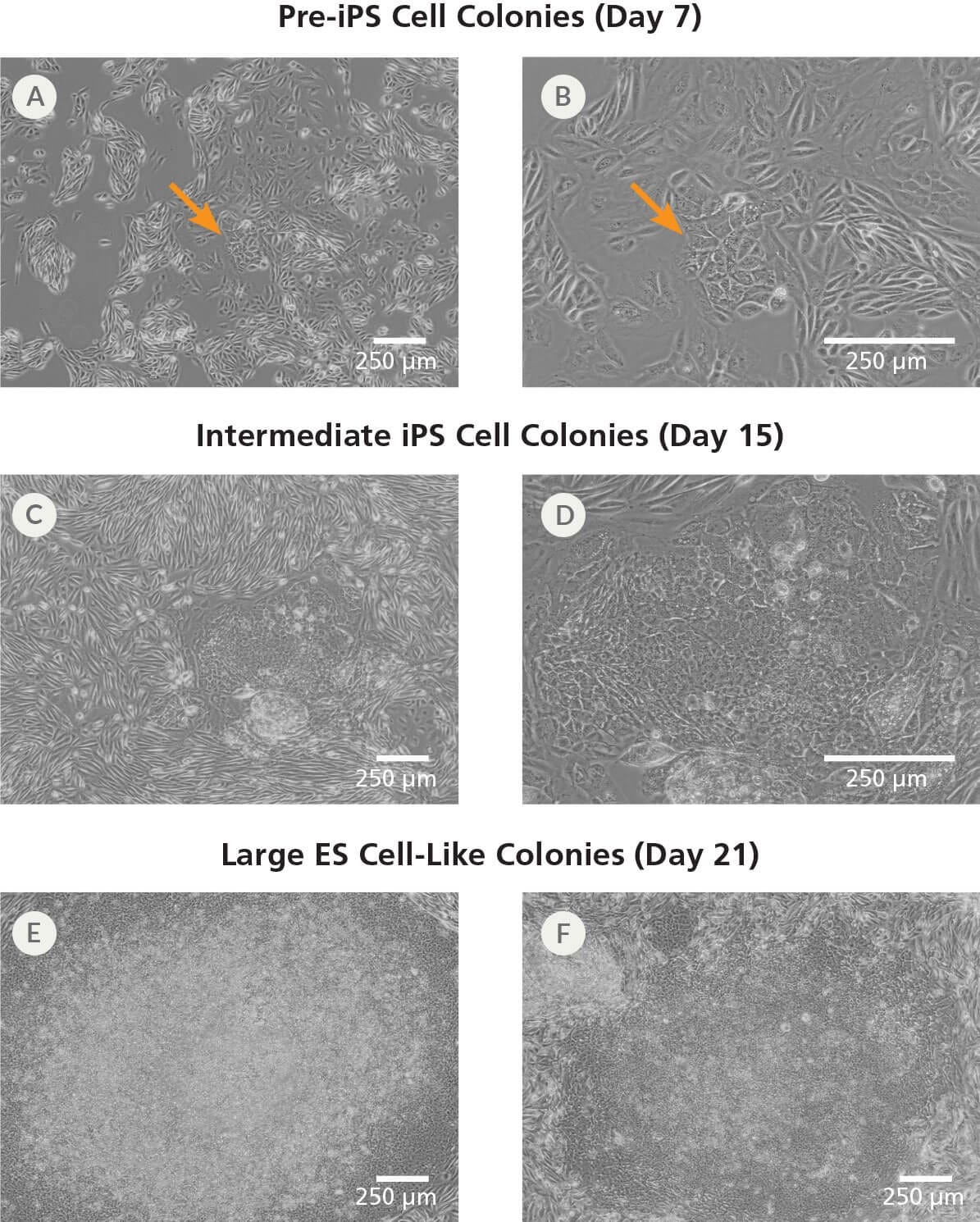
Figure 3. Morphology of iPS Cell Colonies Arising During the Induction Period in ReproTeSR™
(A-B) Small clusters of colonies with a pre-iPS cell morphology will appear by 1 - 2 weeks following induction (see arrows). (C-D) These clusters expand into intermediate iPS cell colonies by 2 - 3 weeks. (E-F) Larger ES cell-like colonies are clearly identifiable by 3 - 4 weeks. Representative images of iPS cell colonies from human UDCs reprogrammed with episomal vectors containing OCT4, SOX2, KLF4, LIN-28, and L-MYC are shown.
D. Measuring Reprogramming Efficiency
At Day 15 - 25, large embryonic stem cell-like colonies will appear
in the reprogramming culture (as seen in Figure 3E - F); at this
stage reprogramming efficiency can be assessed. Reprogramming
efficiency is calculated as a percentage, using a ratio of total iPS cell
colonies and/or areas to the total number of cells seeded in the well
post transfection (section B step 13).

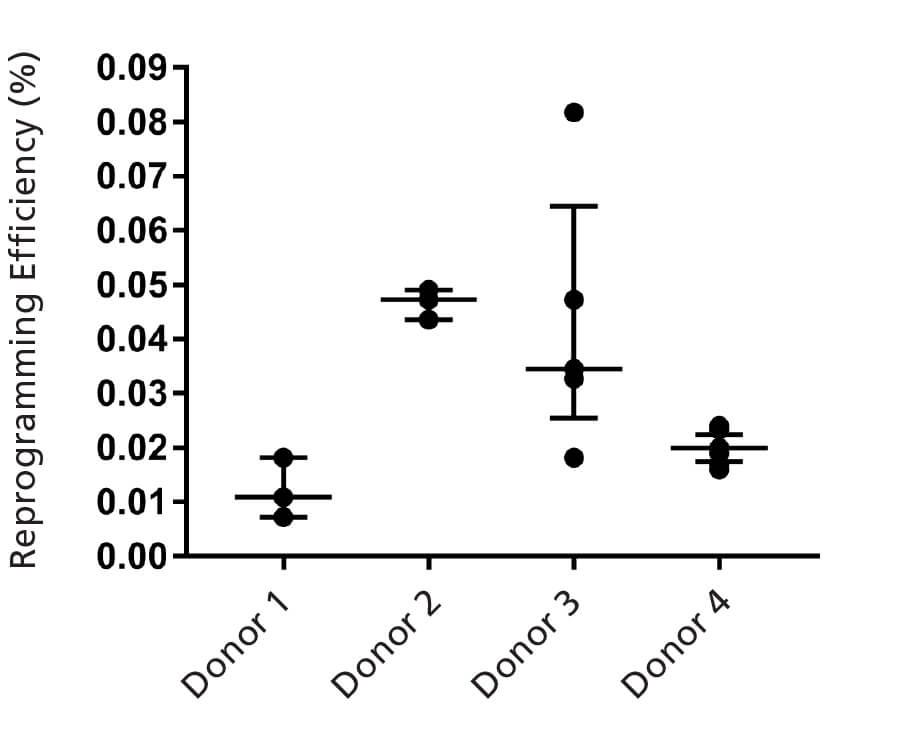
Figure 4. ReproTeSR™ Supports Reprogramming of Human Urine-Derived Cells
Urine-derived cell cultures were established from four separate adult donors (ages 25 - 55 years) and reprogrammed with an episomal vector system containing OCT-4, SOX2, KLF-4, LIN-28, and L- MYC using ReproTeSR™. Data are expressed as median ± interquartile range, n ≥ 3 for each donor sample.
E. Isolating iPS Cell Colonies and Transferring to TeSR™-E8™ or mTeSR™1
At Day 15 - 25, colonies resembling human embryonic stem cell colonies will appear (Figure 3E - F). Once colonies have reached a diameter of approximately 500 - 1000 μm, they can be manually isolated and cultured in a human pluripotent stem cell (hPSC) maintenance medium such as mTeSR™1 or TeSR™-E8™ as described below. Perform the following protocol under a stereomicroscope using sterile conditions.
- Add 2 mL of mTeSR™1 or TeSR™-E8™ to each well of a
6-well plate, pre-coated with Matrigel® or Vitronectin XF™
(Catalog #07180).
NOTE: Only at this first passage, add Y-27632 to culture medium at a final concentration of 10 μM to improve survival of subcloned fragments. - Using either a 22 gauge needle or a pulled glass pipette, separate and scrape away any non-reprogrammed cells such as epithelial cells, partially reprogrammed cells, or differentiated cells, to isolate the putative iPS cell colony.
- Using the needle or pulled glass pipette, cut the putative iPS cell colony into small fragments.
- Using a pipettor with a 200 μL filtered pipette tip, scrape colony fragments to loosen them from the plate and aspirate into tip. Immediately transfer colony fragments to the plate prepared in step 1.
F. Culturing New iPS Cell Lines and Cell Quality Monitoring
For the first 1 - 2 passages, we recommend manual passaging of newly isolated iPS cell lines before adapting to chemical or enzymatic passaging. This can help reduce the presence of contaminating non-reprogrammed or differentiated cells. Once iPS cell lines are established, they can be chemically or enzymatically passaged as described in the Technical Manuals: Maintenance of Human Pluripotent Stem Cells in mTeSR™1 (Document #28315) or TeSR™-E8™ (Document #DX20809), available at www.stemcell.com or contact us to request a copy.
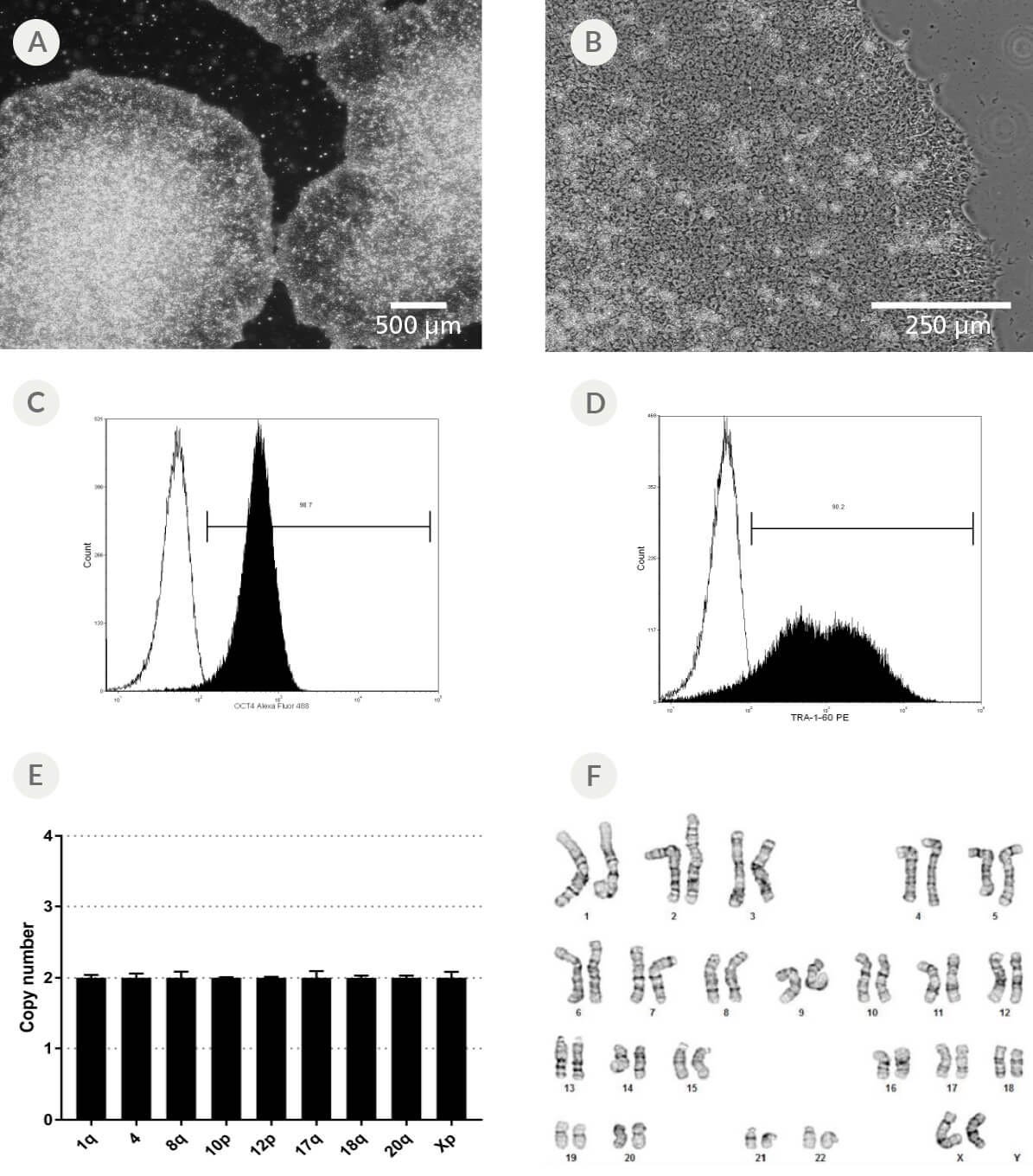
Figure 5. Morphology and Cell Quality of iPS Cell Colonies Expanded in mTeSR™1
(A - D) iPS cell colonies generated in ReproTeSR™ and expanded in mTeSR™1 on Corning® Matrigel® (A-B) exhibit classic ES cell morphology with dense colony centers, defined borders, prominent nucleoli, and high nuclear-to-cytoplasmic ratios. (C - D) iPS cells express high levels of undifferentiated iPSC markers after 9 passages in mTeSR™1 as demonstrated by OCT4 and TRA-1-60 flow cytometry analysis (filled histogram = stained sample, solid line histogram = unstained sample). (E) hPSC Genetic Analysis Kit (Catalog #07550) results obtained at the end of passage 1. Six out of six iPS cell lines tested normal by the hPSC Genetic Analysis Kit. (F) G-banding karyotype analysis performed at the end of passage 7. Two out of two cell lines demonstrated a normal karyotype by g-banding. Representative cell line data are shown.
To ensure high quality of iPS cell lines, we recommend to continually monitor morphology (Figure 5A - B), expression of markers of the undifferentiated state (Figure 5C - D), and pluripotency (Figure 6). To monitor genomic integrity, we recommend using hPSC Genetic Analysis Kit to detect the most commonly reported karyotypic abnormalities in hPSC cultures (Figure 5E), which can be confirmed by Giemsa banding (G-banding; Figure 5F). While hPSC markers such as OCT4 and TRA-1-60 (Figure 5C - D) are expressed by undifferentiated hPSCs, they are not necessarily markers of pluripotency. Rather, hPSC pluripotency can be evaluated through a number of different assays, including in vitro directed differentiation with STEMdiff™ Trilineage Differentiation Kit (Figure 6).
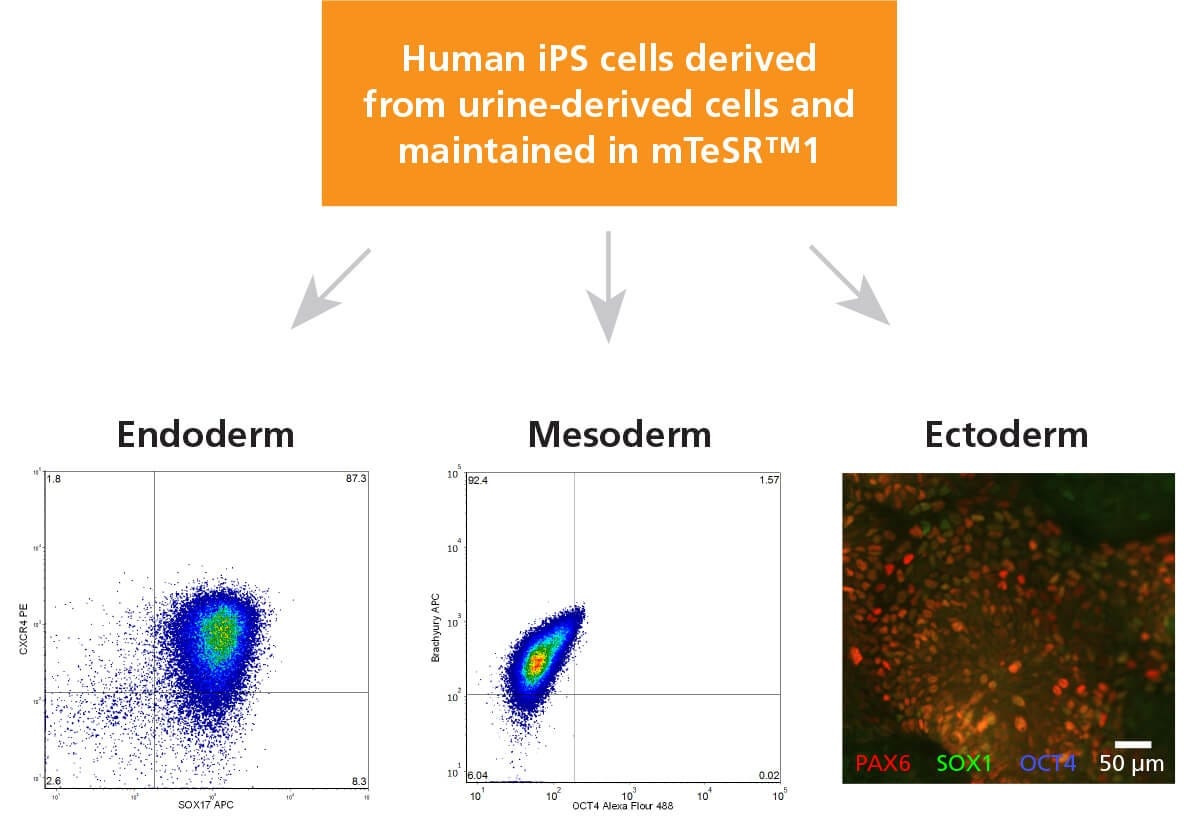
Figure 6. ReproTeSR™-Derived iPS Cells Generated from Urine- Derived Cells Have the Capacity to Differentiate to Cells of the Three Germ Layers
Human iPS cells derived using episomal vector system and maintained in mTeSR™1 for 5 passages were differentiated into cells of the three germ layers. Endoderm specification was achieved using STEMdiff™ Definitive Endoderm Kit (Catalog #05110). Flow cytometry analysis shows a high percentage of cells (87.3%) positive for endoderm markers (CXCR4+ SOX17+). Mesoderm induction was achieved with STEMdiff™ Mesoderm Induction Medium (Catalog #05220) as shown by the high percentage of cells (92.4%) expressing Brachyury (T+OCT4-). Ectoderm specification was demonstrated using STEMdiff™ SMADi Neural Induction Kit (Catalog #08581). Representative immunofluorescence image of PAX6 (red) and SOX1 (green) double positive neural progenitor cells generated from UDC-derived human iPS cells is shown.
References
- Bouma MJ et al. (2017) Differentiation-defective human induced pluripotent stem cells reveal strengths and limitations of the teratoma assay and in vitro pluripotency assays. Stem Cell Reports 8(5): 1340–53.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration

