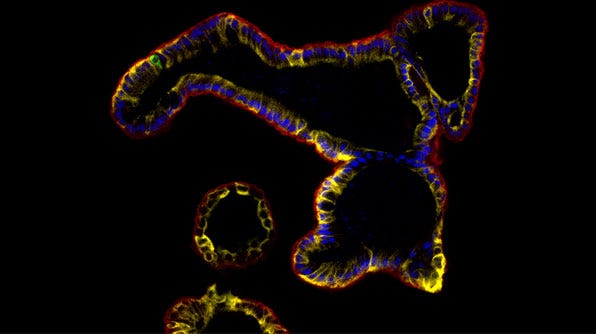
Performing Immunocytochemical Staining of Epithelial Organoids
Using immunocytochemistry (ICC) researchers can detect relevant epithelial markers and assess for markers of differentiation. Besides other end-point characterization studies, ICC staining also enables researchers to evaluate different experimental conditions. For instance, it can be used to assess culture marker expression in the presence or absence of a specific drug or pathogen.
Below, we describe a protocol for whole-mount fixation and immunostaining of epithelial organoids derived from different tissue types including intestinal, mammary, prostate, lung, pancreatic, and liver derived from primary cells or pluripotent stem cells.
Materials
- Normal serum (use the same species as used to generate the secondary antibody)
- Bovine serum albumin (BSA)
- Triton™ X-100
- TWEEN® 20
- D-PBS (Without Ca++ and Mg++; Catalog #37350)
- Axygen® 200µL Pipet Tips, Wide-Bore (Corning®, T-205-WB-C-R-S)
- 4% paraformaldehyde in PBS (PFA)
- DAPI (Catalog #75004)
Preparation
Note: Protocol and volumes are optimized for epithelial organoids (50 - 500 µm) cultured in Corning® Matrigel® (Growth Factor Reduced, Phenol Red Free, Corning Catalog #356231) domes.
- Prepare a 4% PFA Solution by combining the following:
- 10 mL of 16% PFA (e.g. Pierce™ 16% Formaldehyde (w/v), Methanol-free, Thermo Scientific™ Catalog #28908)
- 30mL PBS
Note: 4% PFA Solution may be aliquoted and stored at -20°C for 6 months. Avoid multiple freeze-thaws and exposure to light.
- Prepare Immunofluorescence (IF) Buffer:
- 499 mL PBS
- 500 mg BSA (0.1% w/v)
- 1 mL Triton™ X-100 (0.2% v/v)
- 0.25 mL TWEEN® 20 (0.05% v/v)
Note: IF Buffer may be stored at 2 - 8°C for 6 months.
- Prepare Permeabilization Solution:
- 99 mL PBS
- 1 mL Triton™ X-100 (1% v/v)
Note: Permeabilization Solution without serum may be stored at room temperature for 1 year.
- Prepare Citrate Buffer:
- 1 L dH2O
- 2.94 g Sodium citrate dihydrate
- Mix thoroughly then adjust the pH to 6.0 by adding HCl
- 500 µL TWEEN® 20
Note: Citrate Buffer may be stored at 2 - 8°C for 6 months.
Recovery of Whole Organoids from Matrigel®
Note: This optimization is designed for organoids between 50 and 500 µm in diameter. It is recommended to use at least 100 organoids per panel, some user optimization may be necessary. If the organoids are less than 50 µm in diameter, users will likely need to start with a larger number of organoids. lf the organoids are 1 mm or larger in diameter, this protocol is not appropriate.
Note: Organoid suspensions (i.e. grown in the absence of Matrigel® or with non-gelling Matrigel® concentrations) do not need to be recovered.
- Pre-rinse a 15 mL conical tube with Anti-Adherence Rinsing Solution. Tilt or vortex the tube to ensure the tube wall is coated with rinsing solution.
Note: Pre-rinsing prevents organoid adhesion to cultureware and markedly increases organoid recovery.
- Remove the culture medium from the well and add 1 mL of cold Gentle Cell Dissociation Reagent (GCDR) to the well.
Note: Cold Cultrex® Organoid Harvesting Solution or cold Corning® Cell Recovery Solution (Corning Catalog #354253) can be used in place of GCDR.
- Cut a 1 mL pipette tip (or use a wide-bore pipette tip), and pre-rinse it with Anti-Adherence Rinsing Solution. Use the same tip to (gently) triturate the dome twice, then transfer the organoids to the tube prepared in step 1.
Note: Breaking up the dome into smaller fragments allows for more efficient digestion of Matrigel®. Liberation of organoids from Matrigel® is important to prevent Matrigel® from interfering with downstream staining. Avoid excessive or harsh trituration, which may shear or damage the organoids.
- Place the tube on its side on ice. Place the ice box on a rotating or tilting platform and agitate Matrigel®-organoid suspension for 20 minutes.
- Cut and pre-rinse a 1 mL pipette tip (or use a wide-bore pipette tip) with Anti-Adherence Rinsing Solution and use the same tip to (gently) triturate the Matrigel® fragments.
- Place the tube back on ice and incubate for an additional 20 minutes with agitation.
Note: The incubation is complete when Matrigel® is dissolved and organoids start to float in suspension; this may require an incubation longer than 1 hour.
- Allow the organoids to settle by gravity.
- Aspirate the supernatant, which should contain most of the Matrigel®. Proceed to the organoid fixation step.
Note: If multiplexing, stain only one dome per antibody panel. As an example, if you intend to stain with 4 different panels, pool organoids from 4 domes. Note that depending on the number of the organoids per dome, some optimization may be required. When multiplexing it is important to ensure that the antibody combinations are from different species to avoid cross-reaction during staining.
Fixation
There are two principal methods of chemical fixation: dehydration and cross-linking. Dehydration fixatives (e.g. methanol) remove and replace free water surrounding proteins, causing their denaturation and in situ precipitation. Cross-linking fixatives (e.g. paraformaldehyde) chemically react with proteins to form inter- and intramolecular covalent bridges. Dehydration typically results in higher immunoreactivity, but poor morphology and high protein loss. Cross-linking results in excellent morphological structure but can also lead to epitope masking and antibody exclusion. The optimal fixation method should be determined experimentally for each tissue and for each antibody-antigen interaction. Generally, PFA fixation followed by antigen retrieval leads to the best results.
- Pre-rinse a 1.7 mL microcentrifuge tube with Anti-Adherence Rinsing Solution.
- Remove the supernatant from the organoid pellet and resuspend the organoids in 1 mL of 4% PFA.
- Pre-rinse a 1 mL pipette tip in Anti-Adherence Rinsing Solution and use the same tip to gently transfer the organoids from the 15 mL conical tube to the microcentrifuge tube prepared in step 1.
- Place the tube on its side and incubate in PFA with gentle rocking for 45 minutes.
- After the incubation period, wash the organoids once in IF Buffer to eliminate residual PFA. Let the organoids settle after the wash.
- Resuspend the organoids in 1 mL of PBS and proceed to the Antigen Retrieval Step.
Note: Although the optimal time for fixation will vary between tissues depending on their size and density (and the type of fixative), a fixation time of 45 - 60 minutes will ensure the complete fixation of organoids of most shapes and sizes (i.e. organoids up to 500 µm in diameter).
Note: Performing one wash is generally sufficient. If needed, a second wash may be performed. Allow organoids to settle by gravity after all washes.
Note: Organoids may be stored long term at 2 - 8°C in PBS; however, it is ideal to proceed to the next step as soon as possible, to minimize loss of the antigen signal.
Antigen Retrieval (Optional)
Some fixatives cause cross-linking of epitopes, which masks epitopes and reduces antigen-antibody binding. While antigen retrieval is commonly recommended for formalin-based fixatives, the need will depend on several factors such as target antigen, the antibody used, the type of tissue, and the method and duration of fixation. Antigen retrieval is essential when staining for some antigens (e.g. keratin 8/18) but is optional for other antigens (e.g. Acetylated tubulin). Samples fixed in methanol or other organic solvents should not undergo antigen retrieval.
Note: Antigen retrieval is only meant for samples fixed in PFA or other cross-linking agents. Samples fixed in alcohol or other dehydration-type fixatives should not be boiled.
- Aspirate PBS and add 1 mL of Citrate Buffer to the organoids.
- Set a 1.7 mL-tube heating block to 98°C. Once the heating block reaches 96 - 98°C, place the tube in the heating block and incubate for 20 minutes.
- Turn off the heating block. Allow the organoids to sit in the heating block for an additional 20 minutes while it cools down.
- Proceed to the Permeabilization and Blocking step.
Note: Caution the tubes can be hot at this point. If needed, use tweezers to remove the tubes.
Cell Permeabilization and Blocking
Note: Permeabilization of cells is only needed for intracellular targets. If assessing the surface markers, there is no need for permeabilization. Methanol-fixed samples may not require permeabilization.
- Create a Permeabilization/Blocking Solution by adding 5% serum (v/v) to the Permeabilization Solution.
- Aspirate the Citrate Buffer and add 1 mL of Permeabilization/Blocking Solution to the organoids. Place the tube on its side on a titting platform and incubate at room temperature with agitation for 1 - 72 hours.
Note: The animal serum used for blocking should be the same as the host of the secondary antibody. For example, if you are planning on using donkey secondary antibodies, donkey serum should be used for this step. Add fresh serum each time (i.e. do not store Permeabilization/Blocking Solution long term with serum).
Note: The incubation period may need to be optimized for each tissue, antigen, and antibody. Often, however, the incubation period may range from 1 - 72 hours with little to no difference in background signal.
Note: If the cells have been fixed in PFA, consider washing the organoids with 0.3 M glycine for 30 minutes before blocking. Glycine will bind to unreacted aldehyde groups to quench PFA autofluorescence and to prevent free aldehydes from reacting with primary or secondary antibodies, thereby causing a high background signal.
Primary Staining
- Aspirate the Permeabilization/Blocking Solution and wash 3 times in IF Buffer. Allow the organoids to sit for 5 minutes between each wash.
- Add 0.5 mL - 1 mL of the primary antibody cocktail. Prepare the primary antibodies in IF Buffer.
- Place the tube on its side on a tilting platform and incubate at room temperature for 16 - 72 hours with gentle agitation.
Note: It is highly recommended that some organoids be set aside at this point to be used as an isotype control and/or a secondary only control— Especially if the antigen is rare or the antibody is new.
Note: Typical primary antibody dilutions for organoid whole mount are 1:400 - 1:800; titrate as needed per antibody. User optimization for dilutions may be required depending on the antibody. Use as little antibody as possible to mitigate background signals. Antibody incubation periods may need to be optimized for each tissue, antigen, and/or antibody.
Secondary Staining
Note: Typical antibody dilutions for secondary antibodies are between 1:500 and 1:1000. User optimization may be required depending on the antibody.
- Aspirate the primary antibody solution and wash 3x in IF Buffer. Allow the organoids to sit for 5 minutes between each wash.
- Add 0.5 mL - 1 mL of the secondary antibody cocktail. Prepare the secondary antibodies in IF Buffer supplemented with 10% donkey serum.
- Place the tube on its side on a tilting platform and incubate at room temperature for 16 - 72 hours with gentle agitation. Avoid exposing the organoids to light during this incubation.
Note: The secondary antibody must be raised against the same isotype as the primary. For example, if using mouse IgM as your primary you will need an anti-mouse IgM for your secondary.
Note: At any point after the primary or secondary antibody incubations, the organoids can be stored in IF Buffer (without antibodies) at 2 - 8°C for 2 days or over the weekend (in the dark, without rocking). For best results, continue with the next steps as soon as possible.
Note: To minimize background fluorescence or false signals, it is important that the emission spectra of your conjugated-secondary antibodies do not extensively overlap with one another. Consult the manufacturer's specifications to determine the excitation and emission spectra of your desired fluors.
Counterstaining
- Add DAPI directly to the secondary solution/organoids to a final concentration of 2 - 4 µg/mL. Place the tube on its side and incubate at room temperature with gentle agitation for 15 - 20 minutes in the dark .
- Aspirate the DAPI/secondary solution and wash the organoids once in water and once in PBS. Allow the organoids to sit for 5 minutes between each wash.
Note: If desired, washing samples before the addition of DAPI may be performed, but is not always necessary.
Note: If counterstaining with DAPI, it is best to use red or far-red secondaries to detect nuclear antigens to ensure that any DAPI spillover into downstream channels does not provide a false positive.
Note: At this stage, organoids may be transferred to glass-bottom chamber slides (e.g 8-well chamber slides from Ibidi) and imaged in PBS without clearing. However, clearing is recommended for best results. Organoids may also be stored for 2 - 3 days in PBS at 2 - 8°C, however, it is recommended to image as soon as possible for best results.
Clearing and Mounting
Note: Optical clearing is highly recommended. Not only does the process increase the overall signal-to-noise ratio by reducing light scatter, it also protects fluorophores and dyes from quenching (fading). Immunolabeled and cleared organoids can be stored in ProLong™ Gold Antifade Mountant at room temperature for as long as 6 months with minimal loss in signal intensity.
- Aspirate the supernatant and resuspend the organoid pellet in 1 mL of 50% methanol in PBS. Place the tube on its side on a tilting platform and incubate at room temperature with gentle agitation for at least 1 hour in the dark.
- Remove the 50% methanol solution and add 1 mL of 100% methanol to the organoids. Place the tube on its side on a tilting platform and incubate at room temperature with gentle agitation for at least 1 hour in the dark.
- Use a 1 mL pipette tip followed by a 200 µL pipette tip to aspirate as much of the methanol solution as possible.
- Resuspend the organoid pellet in 50 µL ProLong™ Gold Antifade Mountant.
- Dispense the organoid/mountant suspension onto the centre of a glass microscope slide.
- Place a coverslip on top of the organoid/mountant suspension.
- Allow the mountant to cure for at least 24 hours before imaging.
Note: Mountant is quite viscous. Aspirate and dispense slowly to avoid bubbles. For larger organoids, you may need to cut the 200 µL pipette tip before aspirating.
Note: The weight of the coverslip on the organoids will compress organoids in the Z plane. If you are planning on acquiring Z stacks, you may need to resuspend in a larger volume of mountant (e.g. 100 µL) and transfer the organoids into a chamber slide (e.g. 8-well Ibidi® chamber slide). Alternatively, imaging spacers could be used to confine organoids without compression.
Note: You may want to use clear nail polish to seal the coverslip onto the slide before placing the slide into long-term storage.
Note: In order to reduce light scatter during acquisition, cleared organoids should be imaged in solutions that roughly match the refractive index (RI) of proteins (~1.35-1.6). Prolong™ Gold Antifade Mountant (RI=1.47) is one example of an index-matching solution. Other examples of RI-matching solutions include BABB (2 parts benzyl benzoate with 1 part benzyl alcohol; RI=1.56) and fructose-glycerol (60% glycerol supplemented with 2.5 M fructose; RI=1.469). Keep in mind that organoids immersed in BABB must be imaged on the same day as clearing; organoids immersed in ProLong™ Gold Antifade Mountant may be imaged months after clearing.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration