Nature Research Round Table: HLA Typing Considerations for Human Pluripotent Stem Cell Banking
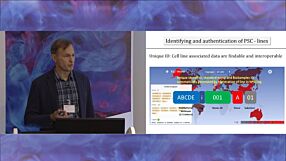
Dr. David Turner from the Scottish National Blood Transfusion Service discusses immunological considerations around banking of human pluripotent stem cell (hPSC) lines for use in clinical trials. Dr. Turner’s work at the Histocompatibility and Immunogenetics (H&I) Laboratory involves donor-patient HLA-matching for clinical transplants. This presentation and the following Q&A session were moderated by Dr. Joanne Mountford from the University of Glasgow.
This presentation was part of a Round Table series titled “Challenges in Ensuring hPSC Quality”, hosted in partnership with Nature Research. Global experts gathered at the Springer Nature headquarters in London, UK, to tackle some of the most pertinent issues impacting the use of human pluripotent stem cells (hPSCs), ranging from fundamental biology research to therapeutic applications. Explore the full series here.
Note: Some original data from this presentation has been omitted to abide by copyright rules.
Dr. David Turner from the Scottish National Blood Transfusion Service discusses immunological considerations around banking of human pluripotent stem cell (hPSC) lines for use in clinical trials. Dr. Turner’s work at the Histocompatibility and Immunogenetics (H&I) Laboratory involves donor-patient HLA-matching for clinical transplants. This presentation and the following Q&A session were moderated by Dr. Joanne Mountford from the University of Glasgow.
This presentation was part of a Round Table series titled “Challenges in Ensuring hPSC Quality”, hosted in partnership with Nature Research. Global experts gathered at the Springer Nature headquarters in London, UK, to tackle some of the most pertinent issues impacting the use of human pluripotent stem cells (hPSCs), ranging from fundamental biology research to therapeutic applications. Explore the full series here.
Note: Some original data from this presentation has been omitted to abide by copyright rules.
This presentation was part of a Round Table series titled “Challenges in Ensuring hPSC Quality”, hosted in partnership with Nature Research. Global experts gathered at the Springer Nature headquarters in London, UK, to tackle some of the most pertinent issues impacting the use of human pluripotent stem cells (hPSCs), ranging from fundamental biology research to therapeutic applications. Explore the full series here.
Note: Some original data from this presentation has been omitted to abide by copyright rules.
Publish Date:
November 01, 2019
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
By submitting this form, you are providing your consent to STEMCELL Technologies Canada Inc. and its subsidiaries and affiliates (“STEMCELL”) to collect and use your information, and send you newsletters and emails in accordance with our privacy policy. Please contact us with any questions that you may have. You can unsubscribe or change your email preferences at any time.