How to Passage Mouse Intestinal Organoids
This protocol is part of a series of protocols for isolating, culturing, passaging, and cryopreserving mouse small intestinal and colonic crypts using IntestiCult™ Organoid Growth Medium (Mouse).
View all mouse intestinal organoid culture protocols >
Learn how to establish mouse intestinal organoid culture before you begin the procedure below.
Materials
- IntestiCult™ Organoid Growth Medium (Mouse) (Catalog #06005)
- Antibiotics (e.g Gentamicyn or Penicillin/Streptomycin)
- Corning® Matrigel® Matrix, Growth Factor Reduced (GFR), Phenol Red-Free (Corning Catalog #356231)
- Gentle Cell Dissociation Reagent (Catalog #100-0485)
- DMEM/F-12 with 15 mM HEPES (e.g. Catalog #36254)
Protocol
Part I: Preparation of Complete IntestiCult™ Organoid Growth Medium
-
Remove the bottle of IntestiCult™ Basal Medium from the refrigerator and place on the bench-top to warm to room temperature (15 - 25°C). Remove the vials of IntestiCult™ Supplement 1 and Supplement 2 from the freezer and leave them to thaw at room temperature (15 - 25°C). Pipette up and down to mix Supplement 1 and 2 thoroughly.
Note: Once thawed, use immediately. -
Make the complete medium by adding 5 mL of Supplement 1 and 5 mL of Supplement 2 to the bottle of Basal Medium. Replace the cap and mix the medium well by inverting the bottle several times.
Note: Complete medium can be stored at 2 - 8°C for up to two weeks. To avoid repeated freeze-thaw cycles, aliquot complete medium into appropriate volumes and freeze at -20°C for up to three months. Do not re-freeze aliquots once thawed. - Immediately before use, add desired antibiotics to complete IntestiCult™ Organoid Growth Medium.
Part II: Passaging Intestinal Organoids
- Add desired antibiotics to the IntestiCult™ Organoid Growth Medium. We recommend 50 μg/mL gentamicin or 100 units/100 μg per mL penicillin/streptomycin.
- Prepare the additional media, buffers and reagents that will be required during the procedure. Thaw 150 μL Matrigel® Matrix per well to be passaged on ice. Also place Gentle Cell Dissociation Reagent and 10 mL DMEM/F-12 with 15 mM HEPES per well to be passaged on ice. Place the required number of tissue culture-treated 24-well culture plates at 37°C to pre-warm for 30 minutes.
- Carefully remove the liquid culture medium from each of the wells to be passaged without disturbing the organoid containing Matrigel® dome.
- Add 1 mL Gentle Cell Dissociation Reagent on top of the exposed dome in each well and incubate at room temperature (15 - 25°C) for one minute.
- Pre-wet a 1000 μL pipette tip with the Gentle Cell Dissociation Reagent in the well, and use it to break up the dome and organoids by pipetting up and down approximately 20 times.
- Use the same pipette tip to transfer the suspension to a 15 mL conical tube. Rinse the culture well with an additional 1 mL Gentle Cell Dissociation Reagent and add this to the 15 mL tube.
- Repeat steps 5 and 6 for each well to be passaged.
- Incubate the 15 mL tubes at room temperature (15 - 25°C) on a rocking platform at 20 rpm for 10 minutes.
- Centrifuge the tubes at 290 x g and 2 - 8°C for five minutes, then gently pour off and discard the supernatant.
- Wash the pellets by resuspending in 10 mL cold (2 - 8°C) DMEM/F-12 using a pre-wetted 10 mL serological pipette, centrifuging at 200 x g at 2 - 8°C for five minutes, then gently pipetting off as much DMEM/F-12 as possible without disturbing the pellet and discarding the supernatant.
- Add 150 μL room temperature complete IntestiCult™ Organoid Growth Medium to the pellet in each tube. Add 150 μL undiluted Matrigel® Matrix to each tube and pipette up and down ten times to resuspend the pellet. Avoid introducing bubbles.
- For each tube, pipette 50 μl of the medium/Matrigel® suspension into the center of each of four wells of a prewarmed 24-well plate to form domes in the center of each well.
- Place the lid on the culture plate and incubate at 37°C for 10 minutes to set the Matrigel®.
- Gently add 750 μL room temperature (15 - 25°C) complete IntestiCult™ Organoid Growth Medium to each well by pipetting the medium gently down the sidewall of the well. Do not pipette the medium directly onto the domed cultures.
- Add sterile PBS to any unused wells.
- Place the lid on the culture plate and incubate at 37°C and 5% CO2.
- Exchange the culture medium three times per week by carefully aspirating the existing liquid medium, keeping the pipette tip at the edge of the well bottom. Replace with 750 μL fresh, room temperature (15 - 25°C) complete IntestiCult™ Organoid Growth Medium.
Using this culture system, organoids can be passaged indefinitely.
Frequently Asked Questions
Can the IntestiCult™ Organoid Growth Medium be thawed in a 37°C water bath rather than at room temperature (15 - 25°C)?
Aliquots of IntestiCult™ Organoid Growth Medium can be thawed in a 37°C water bath before use; however, we do not recommend refreezing medium that has been thawed using this method.
What will happen if I use warm DMEM/F-12 in step 11 of the protocol?
We recommend using cold DMEM/F-12 to wash the dissociated organoid pellets. Using warm medium could result in lower recovery due to cell damage during washing.
What will happen if organoid cultures are plated at a seeding density higher or lower than the recommended 200 organoids per well?
Organoids grow well when near other organoids, yet too high a seeding density can cause strain on the culture. It is not recommended to plate organoid cultures at a density higher than 200 organoids per well; however, we have shown that even at a density of 400 organoids per well, organoids recover within two passages (Figure 1). Organoids plated at a lower seeding density have been shown to recover after one passage.
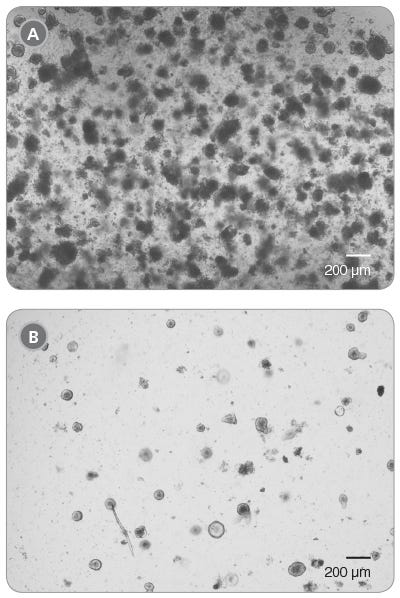
Figure 1. Intestinal Organoid Cultures from Previously Frozen Organoids
Light microscope visualization of organoid cultures when plated at too high a seeding density. Four hundred cryopreserved organoids were thawed, plated in a single 50 μL dome of 1:1 Matrigel® Matrix and IntestiCult™ Organoid Growth Medium and incubated at 37°C and 5% CO2. (A) Passage 0, Day 5 and (B) Passage 1, Day 6.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration