ClonaCell™-CHO ACF Supplement
Animal component-free, serum-free, glutamine-free 40X supplement for cloning during CHO cell line development
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
Overview
Data Figures
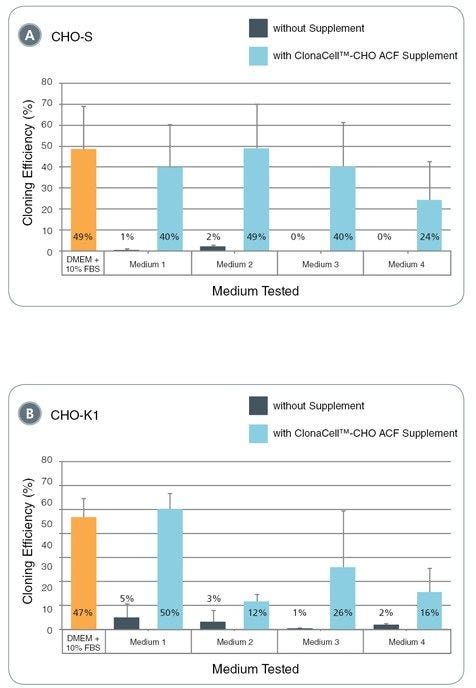
Figure 1. Cloning efficiencies of CHO-S and CHO-K1 cells in protein-free media from different commercial suppliers with and without addition of ClonaCell™-CHO ACF Supplement
The bar graphs illustrate the cloning efficiency of untransfected CHO-S (A) and CHO-K1 (B) cells, defined as the percentage of wells in 96-well plates that contain greater than 100 cells after 14 days of incubation. Individual wells of a 96-well plate were seeded with an average cell density of one cell per well in 200 µL cell culture medium and incubated at 37°C and 5% CO 2. All conditions contained 6 - 8 mM L-glutamine. Cloning efficiencies in various protein-free cell culture media with (blue) or without (grey) addition of ClonaCell™-CHO ACF Supplement or in DMEM + 10% FBS (orange bar) are shown. Error bars represent the standard deviation based on triplicate data for each condition, except Medium 2 (both with and without ClonaCell™-CHO ACF Supplement), for which the average of duplicate data is shown. Medium 1 = ClonaCell™-CHO CD Liquid Medium, Medium 2 = Gibco® CD CHO Medium, Medium 3 = Gibco® CD OptiCHO™ Medium, Medium 4 = Lonza PowerCHO™2.
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Applications
This product is designed for use in the following research area(s) as part of the highlighted workflow stage(s). Explore these workflows to learn more about the other products we offer to support each research area.
Resources and Publications
Educational Materials (8)
Frequently Asked Questions
Are there any selective agents in ClonaCell™-CHO ACF or ClonaCell™-CHO CD?
My cells grow fine in ClonaCell™-CHO ACF but I do not see colony growth in ClonaCell™-CHO CD. Why?
What medium do you recommend for the expansion of clones after selection in ClonaCell™-CHO ACF or ClonaCell™-CHO CD?
How many cells should I plate for cloning in ClonaCell™-CHO CD?
Is the serum in ClonaCell™-TCS medium heat inactivated?
Is there any IgG in ClonaCell™ TCS?
Can ClonaCell™-TCS be used with any cell line?
Why do I get more cells when I select my fusion in liquid medium rather than in methylcellulose-based semi-solid medium?
How do I thaw ClonaCell™ methylcellulose-based semi-solid medium?
How do I measure and dispense methylcellulose semi-solid medium?
My ClonaCell™ methylcellulose semi-solid medium appears runny. Why does this happen?
What is the optimal number of colonies per plate?
There are still bubbles in the media after I plate my cells. Do I need to disrupt the bubbles?
Do I ever need to re-clone cultures grown with ClonaCell™ semi-solid medium?
Once I pick the colonies and grow the cells in plates, will the residual methylcellulose interfere with characterization? For example, will I have problems doing an ELISA?
How important is the incubator humidity when culturing in methylcellulose-based medium?
Do I have to use 100 mm petri dishes or can I use other cultureware?
Related Products
-
 ClonaCell™-CHO CD Medium
ClonaCell™-CHO CD MediumChemically defined, animal component-free, serum-free, protein-free, glutamine-free semi-solid methylcellulose-based medium for selecting and cloning CHO cells
-
 ClonaCell™-CHO ACF Medium
ClonaCell™-CHO ACF MediumAnimal component-free, serum-free, glutamine-free semi-solid methylcellulose-based medium for selecting and cloning CHO cells
-
 ClonaCell™-CHO CD Liquid Medium
ClonaCell™-CHO CD Liquid MediumChemically defined, animal component-free, serum-free, protein-free, glutamine-free liquid medium for culturing CHO cells
-
 ClonaCell™-CHO AOF Supplement
ClonaCell™-CHO AOF SupplementAnimal origin-free, serum-free, L-glutamine-free, and phenol red-free 40X culture supplement for cloning during CHO cell line development
Item added to your cart

ClonaCell™-CHO ACF Supplement
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.