PSC Quality Control
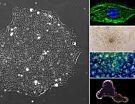
Human embryonic stem (ES) and induced pluripotent stem (iPS) cells are valuable tools for studying human development and disease, as well as a source of differentiated cells for use in regenerative medicine and drug discovery. With the first clinical trials underway, assuring the quality of these cells over long-term maintenance is critical for their safe use in clinical applications.
Products
Cell Culture Media and Supplements 4 (9)
-
eTeSR™
Stabilized, feeder-free medium for single-cell passaging of human pluripotent stem cells -
TeSR™-AOF
cGMP, animal origin-free, stabilized, feeder-free maintenance medium for human ES and iPS cells -
TeSR™-E8™
Feeder-free, animal component-free culture medium for maintenance of human ES and iPS cells -
mTeSR™3D
Serum-free media for suspension culture of human ES and iPS cells
Antibodies 4 (10)
-
Anti-Human TRA-2-54 Antibody, Clone TRA-2-54/2J
Mouse monoclonal IgG1 antibody against human, chimpanzee, gibbon TRA-2-54 (tissue non-specific alkaline phosphatase) -
Anti-Human TRA-2-49 Antibody, Clone TRA-2-49/6E
Mouse monoclonal IgG1 antibody against human, chimpanzee, gibbon TRA-2-49 (tissue non-specific alkaline phosphatase) -
Anti-Mouse OCT4 (OCT3) Antibody, Clone 40
Mouse monoclonal IgG1 antibody against human, mouse OCT4 (OCT3) -
Anti-Human OCT4 (OCT3) Antibody, Clone 3A2A20
Mouse monoclonal IgG2b antibody against human OCT4 (OCT3)
Matrices and Substrates 4 (3)
-
CellAdhere™ Dilution Buffer
Dilution buffer for matrix proteins, e.g. Vitronectin XF™ -
Vitronectin XF™
Defined, xeno-free matrix that supports the growth and differentiation of human pluripotent stem cells under serum-free, feeder-free conditions -
CellAdhere™ Laminin-521
Matrix for maintenance of human ES and iPS cells in combination with TeSR™ maintenance media
Training and Education 4 (1)
-
Pluripotent Stem Cell Training
Training to support the culture of hPSCs and their differentiation towards cerebral organoids, intestinal organoids, cardiomyocytes, or hematopoietic progenitors
Cell Engineering and Molecular Tools 4 (2)
-
hPSC Genetic Analysis Kit
qPCR analysis kit for detecting the majority of karyotypic abnormalities reported in human ES and iPS cells -
Human Pluripotent Stem Cell Trilineage Differentiation qPCR Array
For characterization of hPSC trilineage differentiation to endoderm, mesoderm, and ectoderm germ layers
Cultureware and General Supplies 4 (1)
-
Axygen® Sealing Film for Quantitative Real-Time PCR (qPCR)
Non-sterile, ultra-clear, pressure-sensitive sealing film for quantitative real-time PCR (qPCR)
Tissue and Cell Culture Dissociation Reagents 4 (1)
-
ReLeSR™
cGMP, enzyme-free human pluripotent stem cell selection and passaging reagent