Performing Immunocytochemical Staining of PSC-Derived Kidney Organoids
Using immunocytochemistry (ICC) researchers can detect relevant epithelial markers and assess for markers of differentiation. Besides other end-point characterization studies, ICC staining also enables researchers to evaluate different experimental conditions. For instance, it can be used to assess culture marker expression in the presence or absence of a specific drug or pathogen.
Below, we describe a protocol for fixation and immunostaining of pluripotent stem cells (PSC)-derived kidney organoids using the STEMdiff™ Kidney Organoid Kit (Catalog# 05160). For immunostaining other epithelial cell types, please refer to our protocol Performing Immunocytochemical Staining of Epithelial Organoids.
Materials
- D-PBS (Without Ca++ and Mg++; Catalog #37350)
- Paraformaldehyde (e.g. Affymetrix 199431LT)
- Triton™ X-100 (e.g. Millipore Sigma X100)
- Donkey Serum (eg. Millipore Sigma D9663)
- DAPI (Catalog #75004)
Preparation
Prepare the following reagents before proceeding to fixation and immunostaining:
- Prepare a 4% solution of paraformaldehyde in D-PBS. Mix thoroughly and store at 2 - 8°C.
- Prepare the PBS-T (permeabilization buffer) by diluting Triton™ X-100 in D-PBS to a final concentration of 0.2%. Mix thoroughly.Note: Prepare fresh for each experiment.
- Prepare blocking buffer by diluting Donkey Serum in PBS-T to a final concentration of 10%. Mix thoroughly. Store on ice or at 2 - 8°C while in use.Note: Prepare fresh for each experiment.
- Prepare the DAPI Solution by adding DAPI to Blocking Buffer to a final concentration of 1 µg/mL. Mix thoroughly.
Protocol
- Aspirate medium from the well containing kidney organoids. Carefully add 200 µL D-PBS to the well. Remove D-PBS.
- Add 85 µL 4% Paraformaldehyde Solution to the well (fixation). Incubate at room temperature (15 - 25°C) for 15 minutes. Remove the solution.
- Wash the well 3X with 200 µL D-PBS. Remove D-PBS.
Note: For later immunostaining, keep 200 µL D-PBS in the well, wrap plate with Parafilm®, and store at 2 - 8°C. Remove remaining D-PBS before proceeding to Step 4.
- Add 200 µL PBS-T to the well. Incubate at room temperature (15 - 25°C) for 15 minutes. Remove PBS-T.
- Add 200 µL Blocking Buffer to the well. Incubate at room temperature (15 - 25°C) for at least 30 minutes.
- While incubating Blocking Buffer in the well, prepare primary antibody mix by diluting each primary antibody in Blocking Buffer. Refer to Table 1 for recommended dilutions and antibodies.
- Remove Blocking Buffer from the well and add 80 µL primary antibody mix. Incubate at 2 - 8°C overnight with low shaking.
- Prepare secondary antibody mix by diluting each secondary antibody 1 in 1000 in Blocking Buffer + DAPI (1 µg/mL final concentration). Refer to Table 1 for recommended antibodies.
- Add 200 µL D-PBS to the well and incubate at room temperature (15 - 25°C) for 5 minutes. Remove D-PBS. Repeat this wash step 5X for a total of 6 washes.
- Add 80 µL secondary antibody mix to the well. Incubate in the dark at room temperature (15 - 25°C) overnight with low shaking. Remove secondary antibody mix.
- Add 200 µL D-PBS to the well and incubate at room temperature (15 - 25°C) for 5 minutes. Remove D-PBS. Repeat this wash step 5X for a total of 6 washes.
- Add 200 µL D-PBS to stained cells; they are now ready for immunofluorescent imaging.
Note: If not used immediately for imaging, wrap plate with Parafilm® and store in the dark at 2 - 8°C.
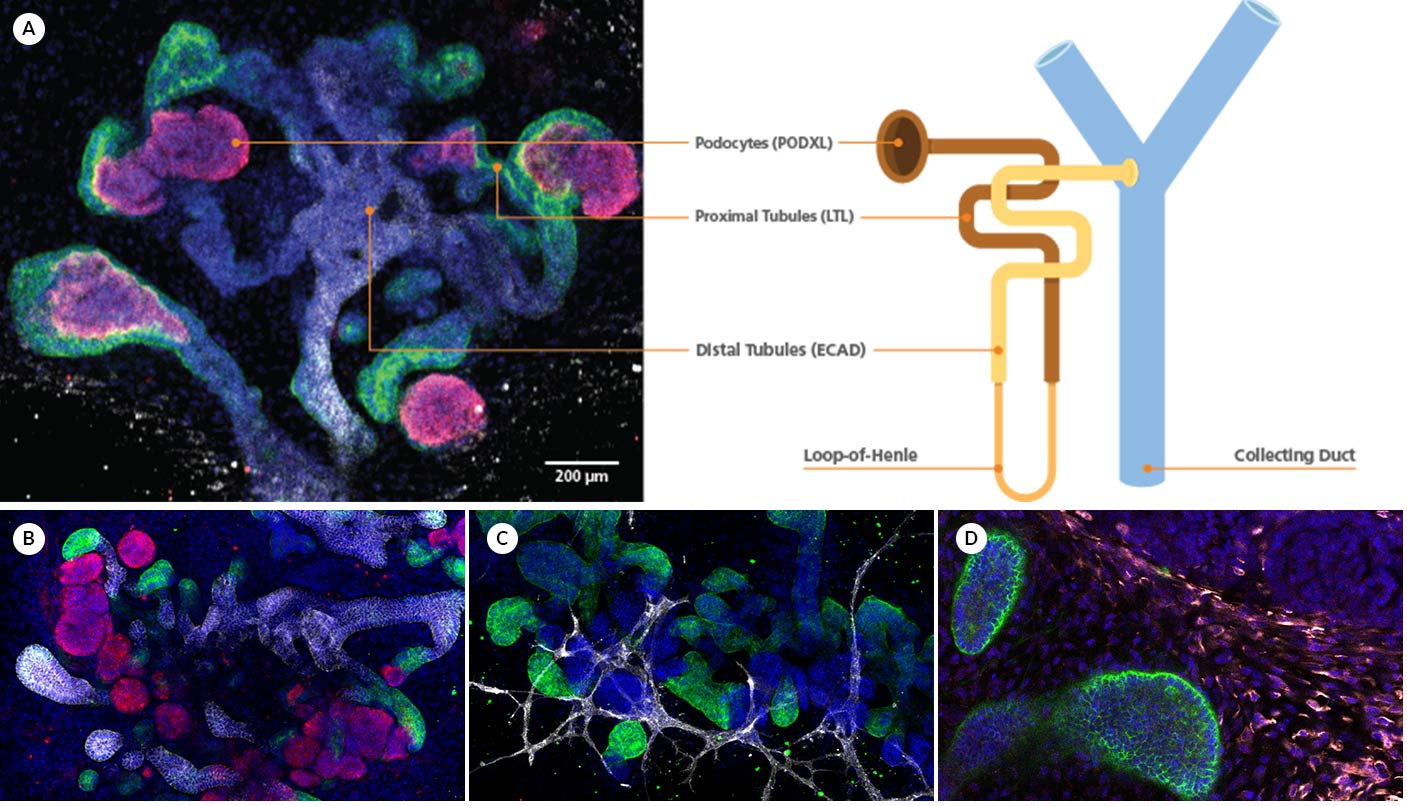
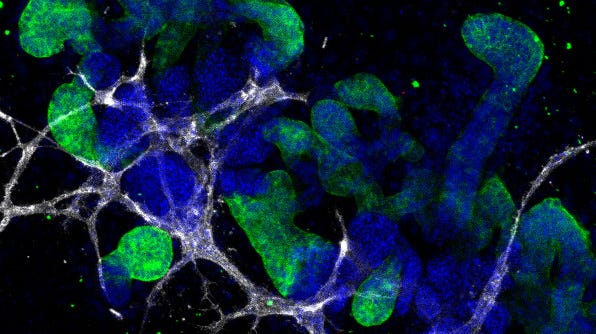
Figure 1. Representative ICC staining data for kidney organoids generated using the STEMdiff™ Kidney Organoid Kit.
(A) During differentiation, kidney organoids form convoluted tubular structures that resemble the structure and segmentation of the developing nephron. These organoids express markers of the (B) renal epithelium including podocalyxin (PODXL), lotus tetragonolobus lectin (LTL), and E-cadherin (ECAD), as well as markers of the (C) endothelium (platelet endothelial cell adhesion molecule, CD31), and (D) mesenchyme (vimentin, VIM; Meis homeobox family, MEIS1/2/3).
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration