How to Remove Granulocytes from Old Blood Samples
How to remove granulocytes by immunodensity cell separation using RosetteSep® Human Granulocyte Depletion Cocktail
Granulocytes change density as blood samples age. This results in granulocyte contamination of mononuclear cells when processing older blood samples (> 24 hours post collection) using a density gradient medium. For effective granulocyte depletion in older human whole peripheral blood samples, immunodensity cell separation can be used to support Lymphoprep™- or Ficoll™-based elimination of granulocytes using density gradient centrifugation.
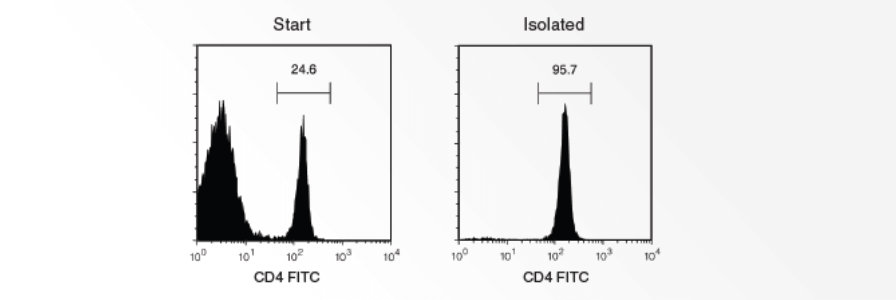
This protocol describes how to remove granulocytes by immunodensity cell separation using RosetteSep® Human Granulocyte Depletion Cocktail. Additionally, a second option is provided in which a SepMate™ tube is used to harvest isolated mononuclear cells with a simple pour. Performing immunodensity cell separation before density gradient centrifugation can result in a blood sample containing < 1% granulocytes, compared to 20% granulocytes with density gradient centrifugation alone.
Option 1: Density Gradient Centrifugation Using a Non-SepMate™ Tube
Materials
- Whole blood sample collected with anticoagulants
- RosetteSep® Human Granulocyte Depletion Cocktail (Catalog #15624)
- Dulbecco's Phosphate Buffered Saline with 2% Fetal Bovine Serum (PBS + 2% FBS, Catalog #07905)
- Lymphoprep™ (Catalog #18060)
- Tube for centrifugation
Protocol
Before You Begin: Ensure that the whole blood sample, PBS + 2% FBS, Lymphoprep™, and centrifuge are all at room temperature (15 - 25ºC).
- Add RosetteSep® Human Granulocyte Depletion Cocktail at 50 µL/mL of whole blood and incubate at room temperature for 20 minutes.
- Dilute whole blood with an equal volume of PBS + 2% FBS and mix gently.
- Layer the diluted sample on top of the Lymphoprep™. Be careful to minimize mixing of the density gradient medium and the sample.
- Centrifuge at 1200 x g for 20 minutes at room temperature (with the brake off).
- Remove the enriched cells from the Lymphoprep™:plasma interface.
- Wash enriched cells with PBS + 2% FBS.
- Repeat wash step.
Option 2: Density Gradient Centrifugation Using a SepMate™ Tube
Materials
- Whole blood sample collected with anticoagulants
- RosetteSep® Human Granulocyte Depletion Cocktail (Catalog #15624)
- Dulbecco's Phosphate Buffered Saline with 2% Fetal Bovine Serum (PBS + 2% FBS, Catalog #07905)
- Lymphoprep™ (Catalog #18060)
- SepMate™-15 (Catalog #85415) or SepMate™-50 (Catalog #85450)
Protocol
Before You Begin: Ensure that the whole blood sample, RosetteSep® Human Granulocyte Depletion Cocktail, PBS + 2% FBS, Lymphoprep™, and centrifuge are all at room temperature (15 - 25ºC).
- Add RosetteSep® Human Granulocyte Depletion Cocktail at 50 µL/mL to whole blood and incubate at room temperature for 10 minutes.
- Dilute whole blood with an equal volume of PBS + 2% FBS and mix gently.
- Add Lymphoprep™ to the SepMate™ tube through the hole in the insert.
- Pipette the diluted sample down the side of the SepMate™ tube.
- Centrifuge at 1200 x g for 20 minutes at room temperature (with brake on).
- Pour off the top layer, which contains the enriched mononuclear cells, into a new tube. Do not hold the SepMate™ tube in the inverted position for longer than 2 seconds.
- Wash enriched cells with PBS + 2% FBS.
- Repeat wash step.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration