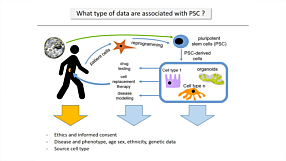
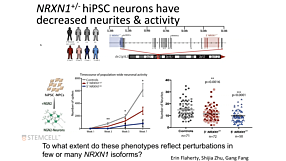
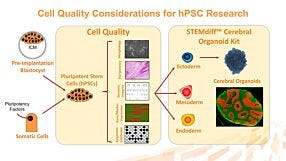
Development, Compatibility, and Applications of mTeSR™ Plus; an Enhanced Medium for the Maintenance of Human Pluripotent Stem Cells (hPSCs)
High quality hPSC maintenance cultures are the foundation for robust and reproducible downstream applications. In this talk, presented at the 2019 ISSCR Annual Meeting in Los Angeles, in-house scientist Dr. Melanie Kardel is joined by Loren Ornelas and Dr. Dhruv Sareen from the Cedars-Sinai Induced Pluripotent Stem Cell (iPSC) Core. This talk features an overview of mTeSR™ Plus, core services, highlights key collaborations, and describes the core facility’s experience with mTeSR™ Plus including cell line transition and quality control.
High quality hPSC maintenance cultures are the foundation for robust and reproducible downstream applications. In this talk, presented at the 2019 ISSCR Annual Meeting in Los Angeles, in-house scientist Dr. Melanie Kardel is joined by Loren Ornelas and Dr. Dhruv Sareen from the Cedars-Sinai Induced Pluripotent Stem Cell (iPSC) Core. This talk features an overview of mTeSR™ Plus, core services, highlights key collaborations, and describes the core facility’s experience with mTeSR™ Plus including cell line transition and quality control.
00:00 - 21:22 Overview of of mTeSR™ Plus presented by Dr. Melanie Kardel
21:22 - 32:05 Cell line transition and quality control at Cedars-Sinai iPSC Core presented by Loren Ornelas
32:05 - 42:10 Cedars-Sinai Biomanufacturing Center presented by Dr. Dhruv Sareen
Publish Date:
August 14, 2019
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
By submitting this form, you are providing your consent to STEMCELL Technologies Canada Inc. and its subsidiaries and affiliates (“STEMCELL”) to collect and use your information, and send you newsletters and emails in accordance with our privacy policy. Please contact us with any questions that you may have. You can unsubscribe or change your email preferences at any time.