Protocol for Semi-Solid Hybridoma Cloning Using ClonaCell™-HY Medium in a 96-Well Plate
.
- Document # 28935
- Version 4.0.1
- May 2023
Reduce the Time Needed to Isolate Producing, Monoclonal Hybridomas
Background
The protocol for semi-solid cloning in 96-well plates combines isolation of discrete colonies, each with a high probability of monoclonality, with the efficiency of selecting and expanding only producing clones. Conventional methods to select and clone antibody-producing hybridomas involve multiple dilution steps in liquid medium. Semisolid cloning using ClonaCell™ methylcellulose-based media from STEMCELL Technologies Inc. reduces the overall time necessary to produce monoclonal cultures by up to 19 days. ClonaCell™-HY media are specifically designed to be a complete solution for hybridoma generation, from cell fusion to hybridoma expansion. The methylcellulose-based semi-solid Medium D contains the selective reagents hypoxanthine, aminopterin and thymidine (HAT), therefore combining HAT selection and cloning of hybridomas into a single step. Using the standard protocol for selection and cloning of hybridomas in ClonaCell™-HY media, fused myeloma cells and splenocytes are suspended in selective semi-solid medium and incubated in 10 cm plates. Individual cells grow to form discrete, monoclonal colonies that are picked from the semi-solid medium after 10 - 14 days of incubation. Colonies are then transferred to individual wells of a 96-well plate and cultured in liquid medium prior to screening the supernatants for clones of interest.
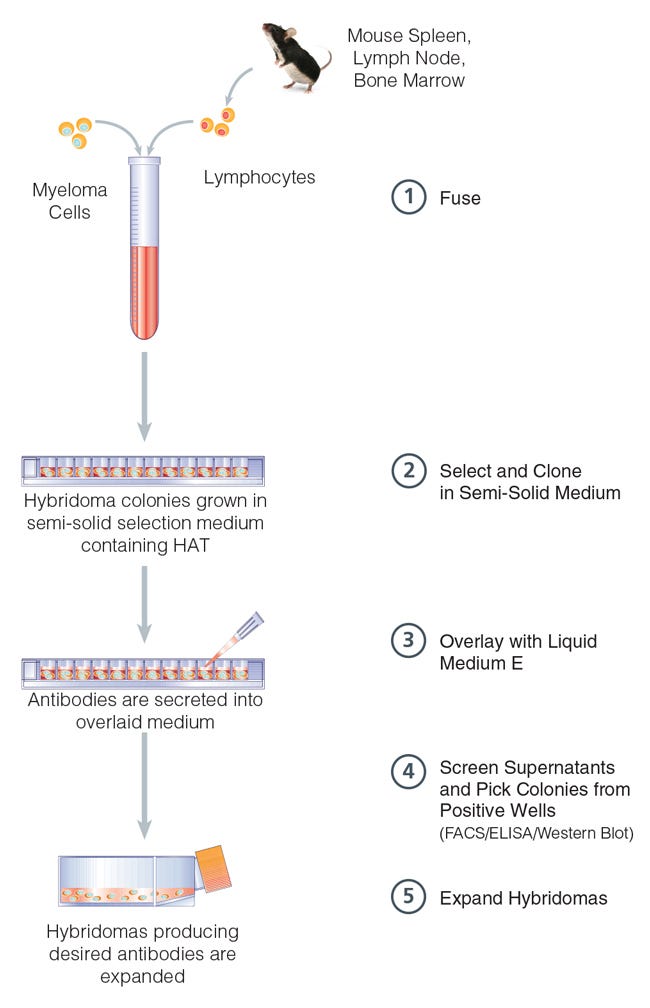
The following technical note describes the use of ClonaCell™-HY Medium D in 96-well plates to reduce the need to harvest and expand large numbers of colonies before screening (Figure 1). Cells suspended in ClonaCell™-HY Medium D are plated directly into wells of a 96-well plate. Cells grow in the semi-solid medium as discrete colonies and secrete antibody products into the surrounding medium. Liquid medium is layered over the semi-solid medium and the secreted antibodies diffuse into the liquid medium, which is then screened to identify colonies producing specific products. This method enables isolation of producing hybridomas with a high probability of monoclonality after only a single round of cloning. These combined benefits result in considerable time and labor savings.
Figure 1. ClonaCell™-HY 96-Well Plate Procedure
Protocol
It is recommended that a fresh vial of myeloma cells be thawed and cultured at least one week prior to the expected fusion date. Ensure myeloma cells are mycoplasma-free. Ideally, the cells used for fusion should be in their logarithmic growth phase.
- On the day of fusion, place the hybridoma semi-solid cloning medium, ClonaCell™-HY Medium D (Catalog #03804), at 2 - 8°C to thaw overnight. Do not place medium in a water bath to thaw.
- Perform fusion of myeloma cells and lymphocytes to yield between 10 - 80 million fused hybridoma cells. For a more detailed fusion protocol, please refer to the ClonaCell™-HY Technical Manual.
- Incubate the fused cells in the recovery medium, ClonaCell™-HY Medium C (Catalog #03803), at 37°C in a humidified, 5% CO2 incubator for 16 - 24 hours.
- On the day after fusion, vigorously shake the thawed Medium D to mix the contents of the bottle and warm to 37°C.
- On the day after fusion, warm the thawed Medium D to 37°C.
- Determine the optimal number of cells to plate per well to arrive at one colony per well. We recommend a range of 10,000 - 80,000 cells/well. If you already have experience with hybridoma selection in liquid HAT medium, plate the same number of cells per well in the semi-solid medium as you would in liquid medium.
- Resuspend the cells in Medium C for a total volume of 10 mL. It is critical not to exceed 10 mL final volume. If you plan to add additional cytokines or growth factors to Medium D, include them with the cells in a total 10 mL volume.
- Combine the 10 mL fused cell suspension with 90 mL Medium D. Mix thoroughly and let sit for 15 minutes to allow the bubbles to rise to the surface.
- Using either a multi-channel pipettor and sterile widebore pipette tips or a repeat pipettor and sterile syringe, dispense 60 - 80 μL of Medium D into each well of a 96-well plate. This will yield between 12 and 16 plates depending on the volume plated. Medium D is a viscous solution and therefore difficult to pipette accurately; however, it is not critical to dispense exactly the same volume into each well.
- Incubate the plates at 37°C in a humidified, 5% CO2 incubator. The incubator should be well-humidified to prevent excessive evaporation. To prevent dehydration, the plates may be placed inside a plastic container that allows proper gas exchange (e.g. 245 mm square treated tissue culture dishes; Catalog #38039/100-0084) along with an open Petri dish containing sterile water.
- Following 8 days of undisturbed incubation, examine wells for the presence of colonies by eye or microscope and gently overlay 150 μL of pre-warmed (37°C) hybridoma growth medium, ClonaCell™-HY Medium E (Catalog #03805), onto the semi-solid medium in each well containing colonies. Alternatively, all wells may be overlaid with 150 μL of pre-warmed Medium E, regardless of the presence of colonies, and analysis is then performed on all wells.
- Incubate plates at 37°C in a humidified, 5% CO2 incubator for an additional 2 - 4 days. The overlay incubation time may be increased further to ensure the detection of low-expressing hybridomas.
- Carefully remove a maximum of 100 μL of the overlaid Medium E without disturbing the colonies in the semi-solid medium. Test the supernatants for specific antibodies using an assay system appropriate for the antigen involved (e.g. ELISA, flow cytometry, western blotting).
- The contents of wells that tested positive for antibodies against the antigen of interest should be gently resuspended and transferred to a single well of a 24-well plate containing 1 mL of Medium E, to expand the hybridomas. If a well contains more than one colony, it may be possible to harvest these clones separately and transfer them to individual wells for expansion and retesting. If wells contain more than one colony and harvesting of individual colonies is not possible, the hybridomas need to be recloned either immediately after harvesting or after a brief 1 - 2 days recovery and expansion period in Medium E. Recloning is not necessary for positive clones which can be harvested independently as these hybridomas have a high probability of being monoclonal. However, it is useful to reclone these hybridomas when selecting for stable, highproducing subclones.
For additional details on hybridoma generation please refer to the Technical Manual for ClonaCell™-HY Hybridoma Cloning Kit (Manual Document #28411).
Time Comparison of Traditional, ClonaCell™-HY, and ClonaCell™-HY 96-Well Protocols for Hybridoma Generation
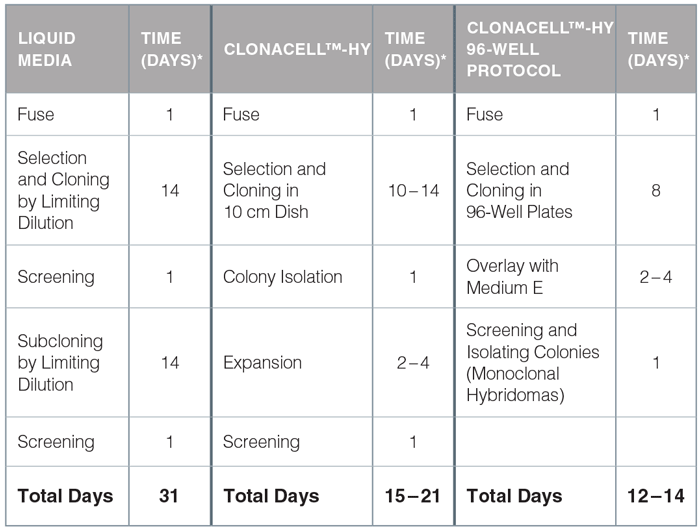
*Estimated times will vary depending on volume of work.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration