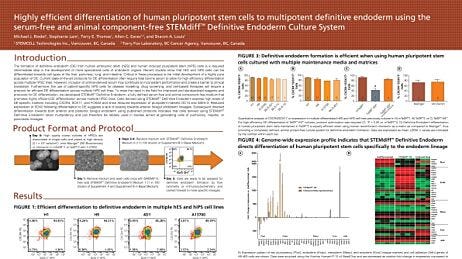
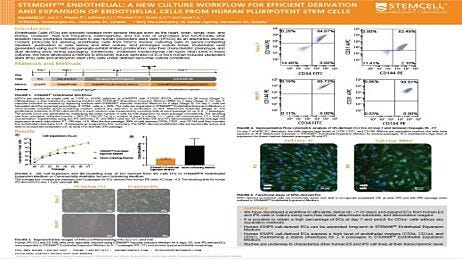
Initiating Intestinal Organoid Differentiation from Single-Cell hPSCs Using STEMdiff™
This protocol describes how to prepare single-cell human pluripotent stem cells (hPSCs) prior to initiating intestinal organoid differentiation with the STEMdiff™ Intestinal Organoid Kit (Catalog #05140). Initiating intestinal organoid differentiation using single cells provides researchers with greater experimental control, consistency, and flexibility because they start with a homogeneous cell population. For instance, differentiating single clones of genetically modified hPSCs can elucidate the role of a specific gene in developmental or disease processes. This protocol can therefore be used to achieve a more homogenous model system to study a range of applications, such as intestinal development and cell biology, intestinal inflammation, intestinal regeneration, microbial interaction, disease modeling, drug discovery, and compound screening. For more information regarding gene editing of hPSCs before initiating downstream differentiation, follow our protocol on Genome Editing of Human Pluripotent Stem Cells Using the ArciTect™ CRISPR-Cas 9 System.
For complete instructions, use this protocol in coordination with the STEMdiff™ Intestinal Organoid Product Information Sheet and the STEMdiff™ Intestinal Organoid Kit Technical Manual, found on the STEMdiff™ Intestinal Organoid Kit Product Page. For more information on maintaining and passaging hPSCs as single cells, please refer to the eTeSR™ page.
Materials and Reagents
- Costar® 24-Well Flat-Bottom Plate, Tissue Culture-Treated (Catalog #38017)
- Corning® Matrigel® Matrix, Growth Factor Reduced (GFR), Phenol Red-Free (e.g. Corning #356231)
- eTeSR™ (Catalog #100-1215)
- Y-27632 (Catalog #72302)
- D-PBS (Without Ca++ and Mg++) (Catalog #37350)
- ACCUTASE™ (Catalog #07920)
- DMEM/F-12 with 15 mM HEPES (Catalog #36254)
- Trypan Blue (Catalog #07050)
Protocol
Day -2, Prior to Stage 1
- Coat a 24-well tissue culture-treated plate with Corning® Matrigel® (see Section 3.0 in the STEMdiff™ Intestinal Organoid Kit Technical Manual).
- Aliquot sufficient eTeSR™ medium and warm to room temperature (15 - 25°C).
NOTE: Do not warm eTeSR™ medium in a 37°C water bath.
- Add Y-27632 to eTeSR™ medium to a final concentration of 10 μM. In addition, set aside warm eTeSR™ medium without Y-27632.
- Use a microscope (e.g. 4X magnification) to visually identify regions of differentiation in your hPSC cultures. Mark these using a felt tip or lens marker on the bottom of the plate.
- Remove regions of differentiation by scraping with a pipette tip or by aspiration. Avoid having the culture plate out of the incubator for more than 15 minutes at a time. Aspirate remaining medium.
NOTE: Removal of differentiated cells will result in better differentiation efficiency.
- Wash the wells to be passaged with 1 mL of D-PBS (Without Ca++ and Mg++).
- Aspirate the wash medium and add 0.5 mL ACCUTASE™ to the wells. Incubate at 37°C for 6 minutes.
NOTE: The incubation time may vary depending on the cell line or cell passaging reagent used.
- Visually ensure that cells start to lift off by gently tapping against the side of the culture plate. Add 1 mL eTeSR™ medium without Y-27632 to each well. Carefully pipette the cell mixture up and down to break up the colonies using either a 1 mL pipette or a 2 mL serological pipette to obtain a single-cell suspension.
- Combine the cell mixtures in a 50 mL conical tube. Rinse each well with 5 mL of DMEM/F-12 with 15 mM HEPES and add the rinse to the tube containing the cells.
Optional: Residual clumps of cells can be removed by passing the cell suspension through a 37 μm Reversible Strainer and retaining the filtrate.
- Centrifuge cells at 300 x g for 5 minutes at room temperature (15 - 25°C).
- Remove and discard the supernatant. Resuspend the pellet in a small volume, sufficient for cell counting, of eTeSR™ medium supplemented with 10 μM Y-27632.
- Dilute a sample (e.g. 10 μL) of the cell suspension 1 in 10 in Trypan Blue and mix gently. Count viable, unstained cells using a hemocytometer.
For more information, refer to the Product Information Sheet for Trypan Blue available at www.stemcell.com or contact us to request a copy.
- Calculate the viable cell concentration as follows:
Viable cell count per mL = (average count per square) x 10 x 104 - Add the appropriate volume for 20,000 - 30,000 viable cells to a 24-well plate (prepared in Step 1) containing 0.5 mL eTeSR™ medium supplemented with Y-27632 per well. Incubate at 37°C with 5% CO2 and 95% humidity. Ensure cells are evenly distributed within each well by rocking the plate in a back-and-forth and side-to-side motion a few times while the plate is in the incubator. Do not disturb the plate for 24 hours.
NOTE: Single-cell seeding density can be optimized using different cell lines by seeding a range of cell densities (e.g. 10,000, 20,000, 30,000 single cells per well). Initiate differentiation of varying density ranges on the same day in order to evaluate optimal cell seeding density.
- Proceed to Section 6.0 in the STEMdiff™ Intestinal Organoid Kit Technical Manual for intestinal organoid differentiation of your single-cell population.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration