Extracellular Vesicle Generation from Mesenchymal Stromal Cells Using MesenCult™-ACF Plus
- Document # 27178
- Version 1.1.0
- Dec 2023
This technical bulletin describes a protocol for generating extracellular vesicles using MesenCult™-ACF Plus Medium which enables extracellular vesicle (EV)-free culture of mesenchymal stromal cells (also known as mesenchymal stem cells; MSCs). MesenCult™-ACF Plus is animal component- and EV-free and is optimized to derive human MSCs from multiple sources, including bone marrow and adipose tissue. The small EVs generated using this medium are fully functional as indicated by the endothelial tube formation assay. The lack of contaminating EVs in this media leads to reduced experimental variability and results in homogeneous samples when generating MSC-EVs.
Why Use MesenCult™-ACF Plus for Generating Extracellular Vesicles?
- Achieve superior cell expansion compared to serum-containing or EV-depleted serum-containing media.
- Use cultured MSCs to generate functional EVs capable of enhancing angiogenesis.
- Derive MSCs directly from primary human tissue without the addition of animal-derived components.
MesenCult™-ACF Plus: An EV-Free MSC Culture Medium
To confirm that MesenCult™-ACF Plus is EV-free, the complete medium particle concentration and properties were investigated using several methods. MesenCult™-ACF Plus particle concentration was compared to fetal bovine serum (FBS)- or human platelet lysate (hPL)-containing media (Figure 1). Nanoparticle tracking analysis of different batches from each medium detected at least 94% fewer nanoparticles in MesenCult™-ACF Plus compared to FBS- and hPL-containing media. MesenCult™-ACF Plus was also tested for EV-specific markers using Western blotting. The commonly used EV markers, CD9, CD63, and CD81, were not present in MesenCult™-ACF Plus at detectable levels (Figure 2).
MesenCult™-ACF Plus was also compared to EV-depleted FBS. Nanoparticle tracking analysis indicated that MesenCult™-ACF Plus had a similar particle concentration to EV-depleted FBS-supplemented media (Figure 3A) and led to superior MSC expansion when compared to FBS-containing medium or medium supplemented with EV-depleted FBS (Figure 3B).
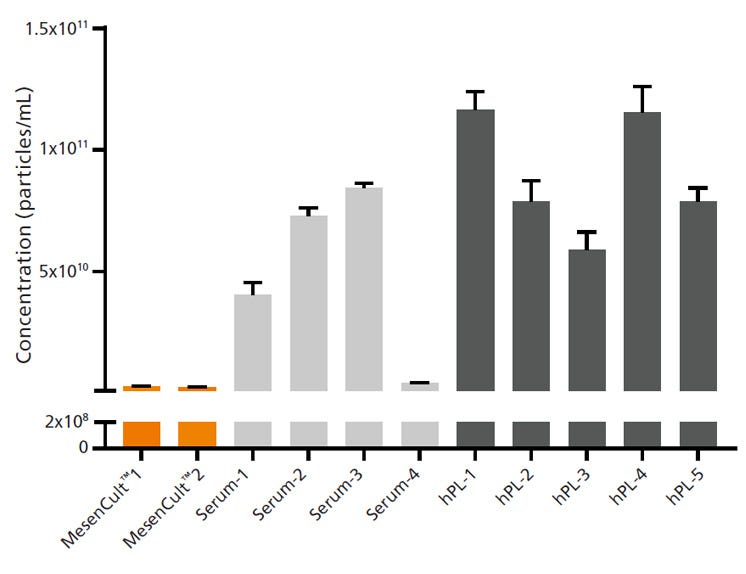
Figure 1. Complete MesenCult™-ACF Plus Medium Contains at Least 90% Fewer Nanoparticles than Serum- and hPL-Containing Media
Nanoparticle tracking analysis was used to demonstrate particle concentration in complete media made with 10% v/v of 4 different lots of serum (FBS), 5 different lots of hPL, or 2 different lots of MesenCult™-ACF Plus. Media containing serum or hPL had high concentrations of nanoparticles with high batch-to-batch variability. MesenCult™-ACF Plus had a significantly lower number of particles/mL when compared to serum- or hPL-containing media. When quantified, particle concentration in each MesenCult™-ACF Plus lot had less than 6% of average serum- or hPL-containing medium nanoparticle concentration. Error bars represent standard error of mean (SEM; n = 3).
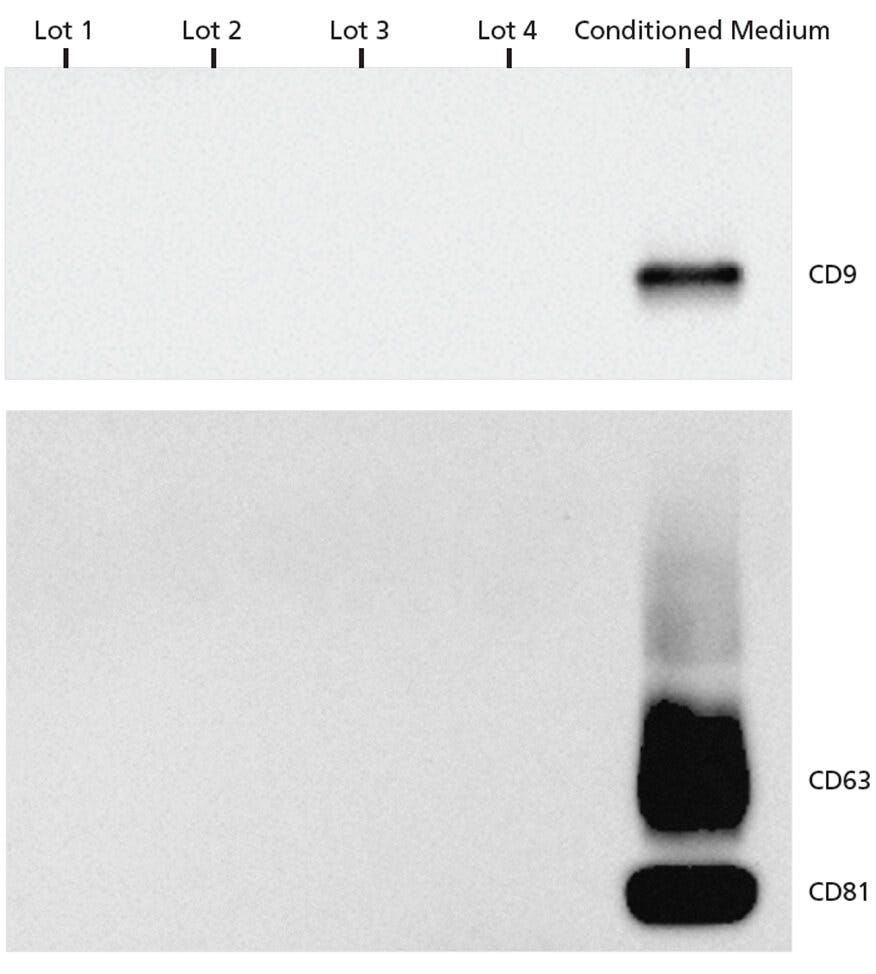
Figure 2. EV Markers Are Undetectable in Complete MesenCult™-ACF Plus Medium by Western Blot Analysis
Western blot analysis was used to investigate 4 different lots of MesenCult™-ACF Plus complete medium for the presence of EVs. Conditioned MSC-cultured MesenCult™-ACF Plus Medium was used as positive control. EV markers, CD63, CD81, and CD9 were only observed in conditioned medium and not in any of the unconditioned MesenCult™-ACF Plus lots, suggesting that this medium is EV-free. 2.3 x 107, 1.9 x 108, 2.7 x 107, 2.5 x 108, and 4.6 x 108 nanoparticles/well were loaded for MesenCult™-ACF Plus lots 1, 2, 3, 4, and the conditioned medium sample respectively.
MSCs Cultured in MesenCult™-ACF Plus Medium Generate Functional EVs
Since MesenCult™-ACF Plus is animal component- and EV-free, it is an ideal medium for generation of EVs from MSCs. The EV-free nature of MesenCult™-ACF Plus ensures the absence of contaminating EVs. MSC expansion and EV generation is not compromised using this medium. To confirm this, the EVs derived using MesenCult™-ACF Plus were assayed for functionality using a human umbilical vein endothelial cell (HUVEC) tube formation assay (Figure 4). Bioactive molecules, including miRNAs, produced by MSCs and packaged into EVs have been shown to promote angiogenesis in endothelial cultures1,2, making this assay a suitable method to test MSC-EV functionality. HUVECs treated with EVs isolated from MSCs cultured in MesenCult™-ACF Plus exhibited enhanced angiogenesis with an increased number of branch points. In contrast, HUVECS not treated with MSC-EVs formed fewer branch points and at slower rates (Figure 4). The cargoes carried by MSC-EVs produced in MesenCult™-ACF Plus were analyzed by qPCR. A high abundance of EV-specific markers, let7, miR21, and miR26a, was observed in EVs derived in MesenCult™-ACF Plus (Figure 5).
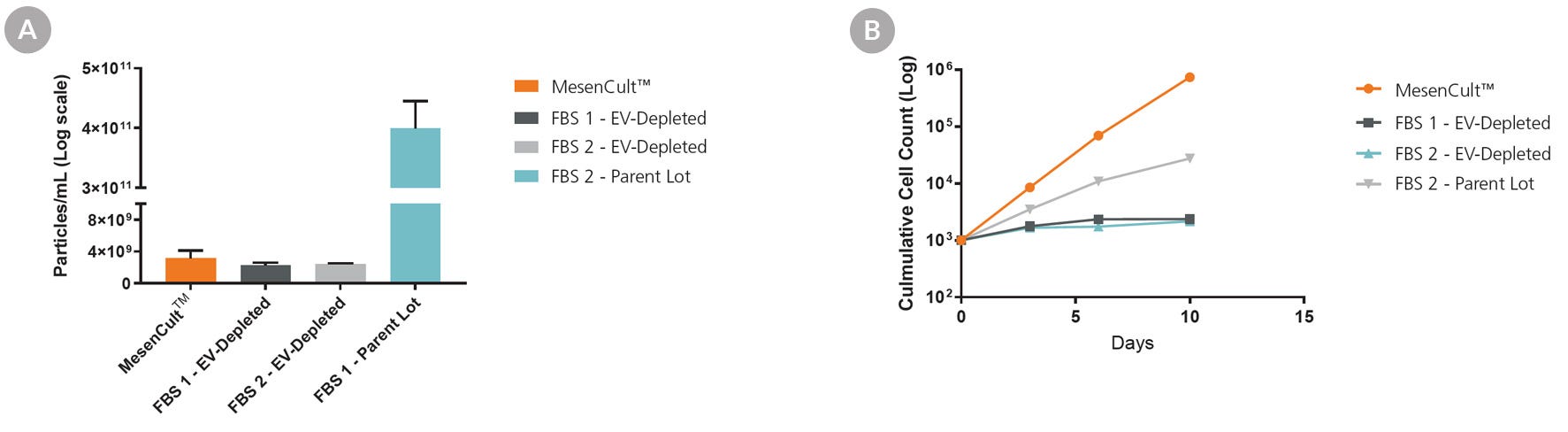
Figure 3. Complete MesenCult™-ACF Plus Medium Performs Similarly or Better than EV-Depleted FBS-Supplemented Medium
Commercial EV-depleted FBS (FBS 1 and FBS 2) was compared to MesenCult™-ACF Plus complete medium. FBS 1 parent lot (non-EV-depleted) was also used for comparison. (A) Particle concentration was measured in complete MesenCult™-ACF Plus and the FBS-supplemented media using nanoparticle tracking analysis. (B) MSC proliferation in MesenCult™-ACF Plus Medium was compared to media supplemented with EV-depleted FBS-supplemented media. The complete MesenCult™-ACF Plus medium had similar nanoparticle concentration to the FBS-depleted media and led to superior cell proliferation compared to FBS-containing or EV-depleted FBS-supplemented media. Error bars represent SEM (n = 3).
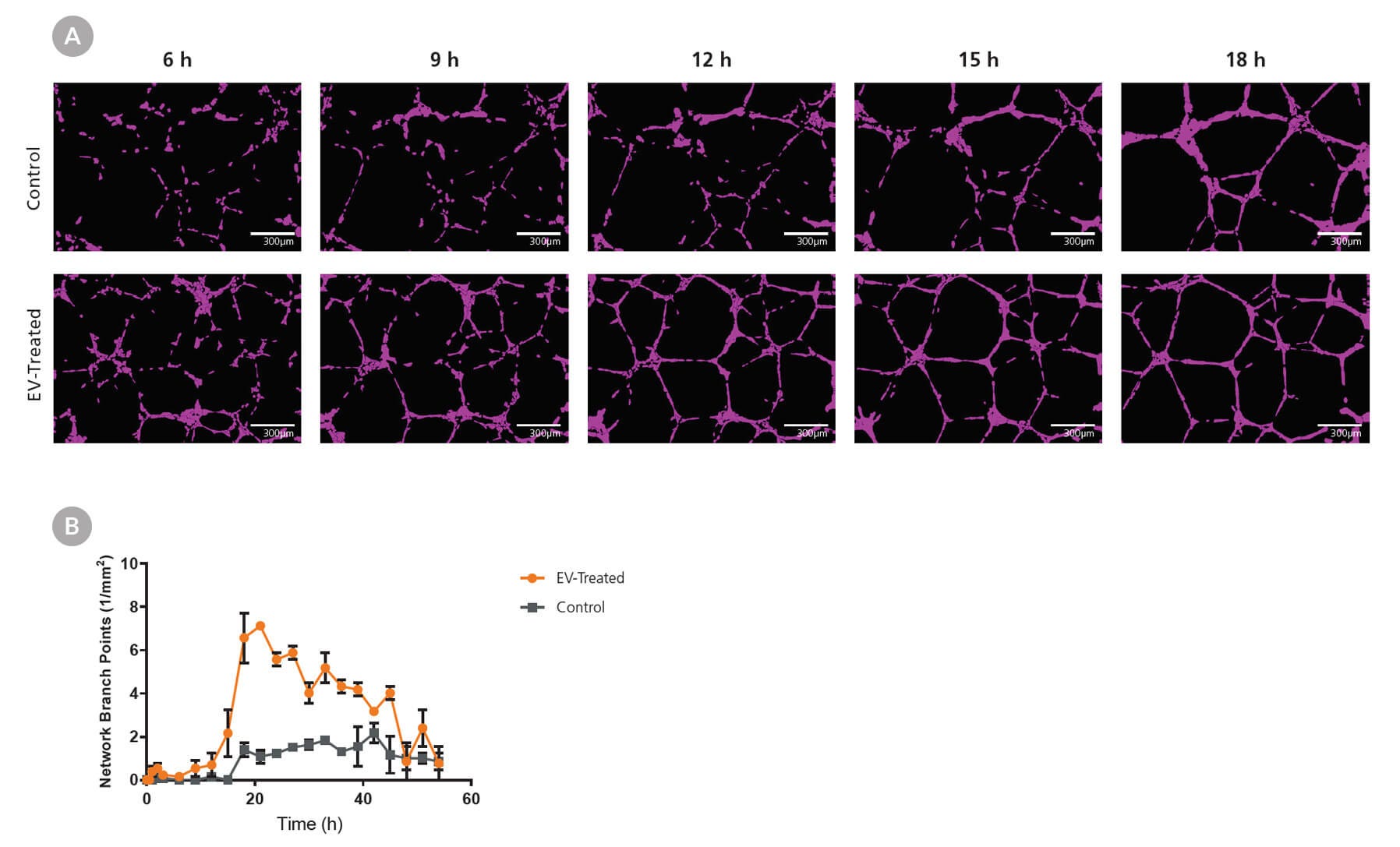
Figure 4. MesenCult™-ACF Plus-Generated EVs Enhance Angiogenesis in HUVECs
MSCs were cultured in MesenCult™-ACF Plus complete medium and EVs were isolated subsequently. Human umbilical vein endothelial cells (HUVECs) were plated at 10,000 cells/well (Matrigel®-coated 96 well plate) in xeno-free endothelial media and treated with 2.6 x 109 particles/well. Formation of tubular networks was then (A) imaged and (B) quantified over the course of 54 hours. The EV-treated group formed a larger number of tubular networks compared to the untreated control group. Error bars represent SEM (n = 3).
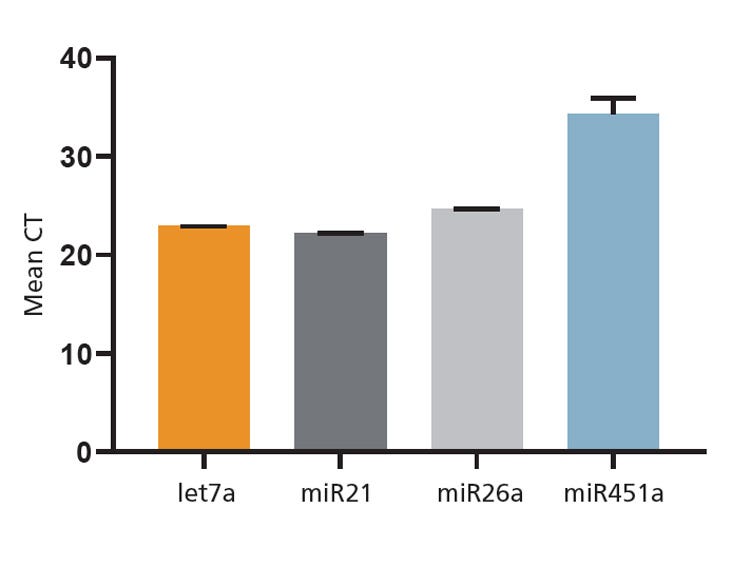
Figure 5. Cargo Analysis of EVs Generated in MesenCult™-ACF Plus Medium
EVs isolated from MSCs cultured in MesenCult™-ACF Plus were analyzed using RT-qPCR and EV-specific mRNA and miRNA cargoes were detected. Error bars represent SEM (n = 3).
Protocol
EV Isolation From MSCs
MSCs may be derived from bone marrow, umbilical cord, fat, or other tissues. We recommend using an EV-free medium for MSC derivation and expansion to avoid external EV contamination. Following the derivation and expansion of MSCs, EVs can be isolated from MSCs using the methods described below. For details on the maintenance and expansion of MSCs, refer to the product information sheet (PIS) for MesenCult™-ACF Plus Medium (Document #10000003462). A schematic overview of the isolation process can be found in Figure 6.
- Culture MSCs in MesenCult™-ACF Plus according to the PIS (Document #10000003462). Once 100% confluent, change the medium and allow 3 - 4 days for EV production by MSCs.
- Transfer conditioned medium to an appropriately sized centrifuge tube.
- Centrifuge for 10 minutes at 2000 x g at 4°C to remove cells and large debris.
- Pipette the supernatant into an ultracentrifuge tube.
Note: Do not decant the supernatant; always use a pipette. Leave behind some liquid above the pellet to avoid contamination. The cell pellet may not be visible at this step. - Ultracentrifuge for 30 minutes at 10,000 x g at 4°C to remove small debris and organelles, ensuring preparation purity and eliminating sources of material contamination.
- Without disrupting the pellet, pipette the supernatant into new tubes. The cell pellet may not be visible at this step.
Note: For fixed-angle rotors, the pellet is on the side of the tube facing out from the center of the rotor, near the bottom of the tube. When working with this setting, pour off the supernatant; do not use a pipette. For swinging-bucket rotors, the pellet is in the center of the bottom of the tube. When removing the supernatant, hold the tube at an angle so that the pellet is always covered with liquid. Stop removing the supernatant when 0.5 cm liquid is still covering the pellet location. - Ultracentrifuge for 70 minutes at 100,000 x g at 4°C.
- Remove and discard the supernatant. The cell pellet may not be visible at this step.
- Add cold PBS to fill the tube completely.
- Ultracentrifuge for 60 minutes at 100,000 x g at 4°C.
- Remove and discard the supernatant, leaving ~200 μL in the vial and add. Then add 200 - 400 μL of cold PBS for a total volume of ~500 μL, resuspend the pellet, and keep on ice.
Optional Step: To further purify the EVs, refer to the next section: EV Purification Following Differential Centrifugation. Although EVs can be stored or used without this optional step, they may have more contaminants if these additional steps are not performed.
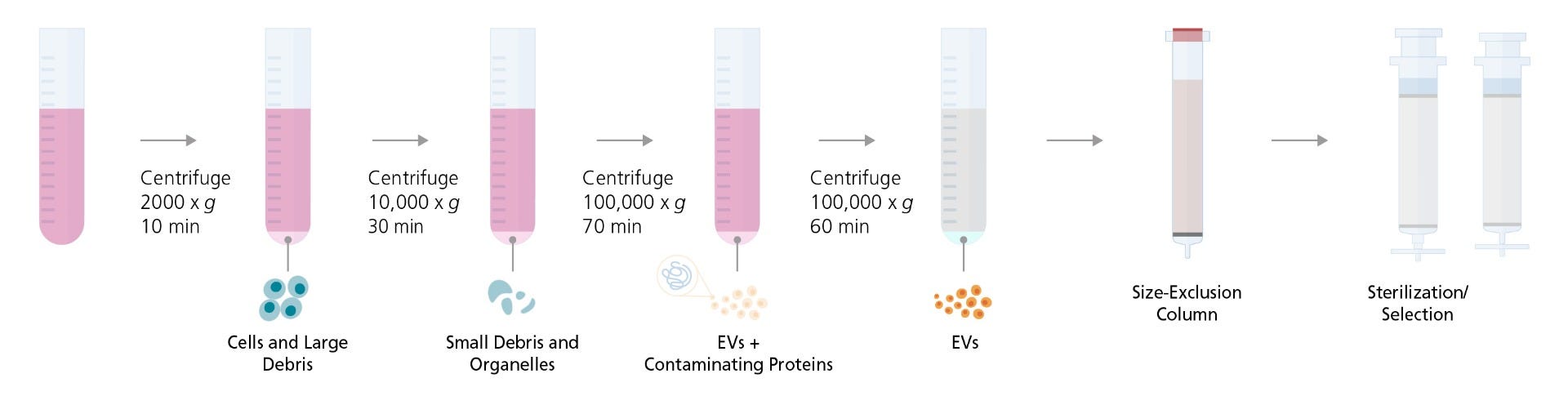
Figure 6. A Schematic Overview of EV Isolation and Purification Method
EV Purification Following Differential Centrifugation
EVs isolated by differential ultracentrifugation can be further purified using size-exclusion columns and sterilized by filtration (Figure 6). These extra purification steps lead to small-EV samples with minimal protein contamination (Figure 7).
NOTE: Perform all steps at 4°C
- Add the resuspended pellet from the ultracentrifugation step to an EV size-exclusion column.
- Gradually add PBS (up to 20 mL) to the column and collect 500 μL fractions.
- Combine 5 fractions from fractions 7 - 12 for a total of ~2.5 mL and discard the others.
- Pre-wash a 0.2 μm polyethersulfone (PES) membrane syringe filter with 2 mL of PBS and discard flow-through.
- Pass the 2.5 mL EV solution from Step 6 of the EV Isolation from MSCs protocol through the syringe filter.
- Wash the filter with 2.5 mL of PBS to collect retained EVs and add to the EV solution from Step 5 of this protocol for a total of 5 mL.
Note: This step is required if you intend to measure the total EV recovered. Skip this step to achieve a more concentrated EV sample for assays. - Analyze the small EVs and use them immediately, or store them at 4°C for short-term storage or -80°C for long-term storage. Avoid repeated freeze-thaw cycles.
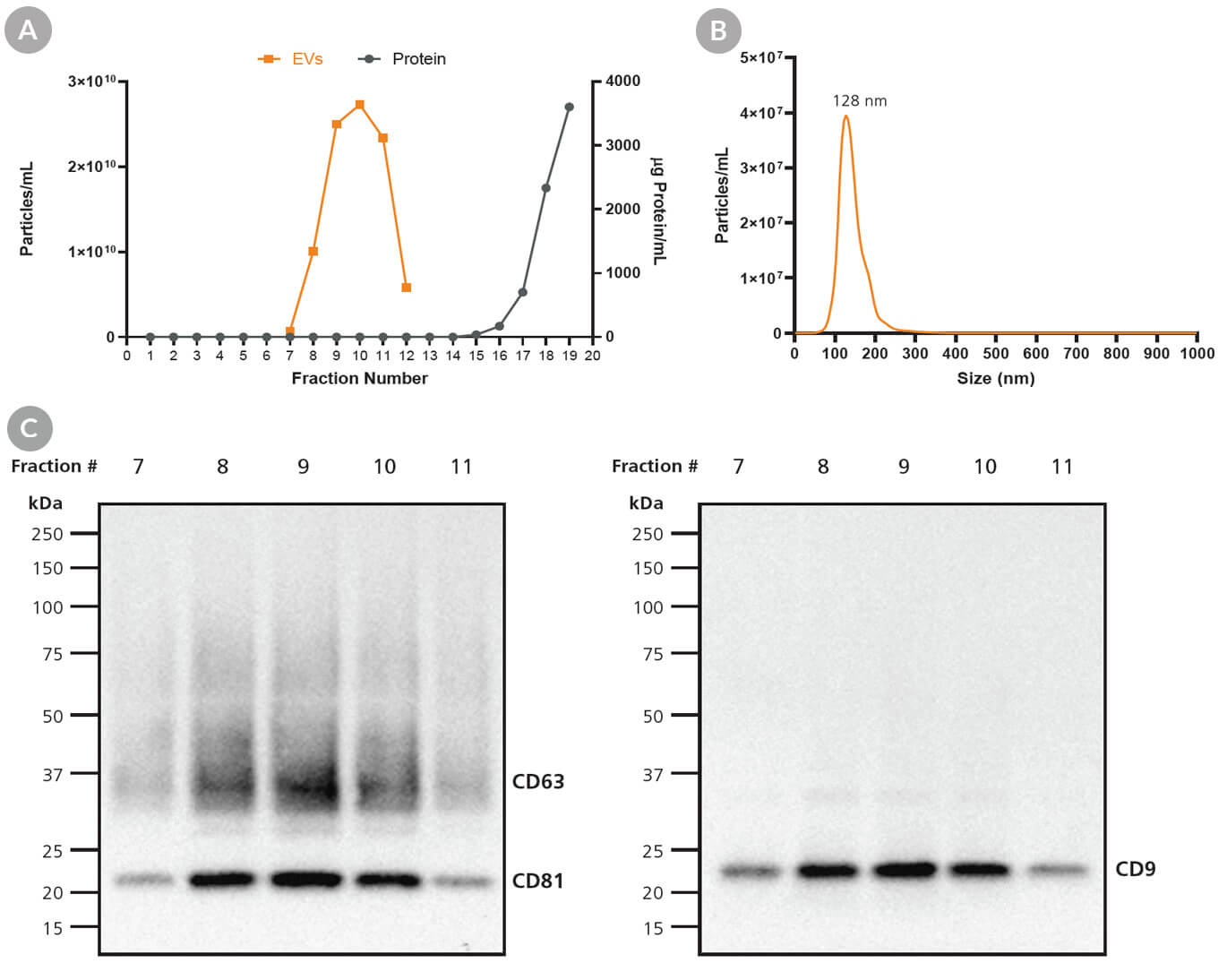
Figure 7. Purification and Sterilization of Exosomes Derived Using MesenCult™-ACF Plus
EVs were isolated from MSCs following a 3 - 4 day culture period using differential chromatography and were further purified using size-exclusion chromatography as previously described. (A) Fractions highly enriched in EVs can be selected and pooled to achieve EV samples with (B) characteristic EV size and (C) EV markers, CD9, CD63, and CD81.
Explore these Resources
References
- Liang X et al. (2016) Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J Cell Sci 129(11): 2182–9.
- Gong M et al. (2017) Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 8(28): 45200–12.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration