How to Isolate Antigen-Specific T Cells
The isolation of antigen-specific T cells has long been of interest to immunologists in vaccine, infectious disease, immuno-oncology and immunotherapy research.
Antigen-specific T cells can be isolated using two methods:
1. Activation-Induced Markers (AIM) Method
This method is based on the concept that an antigen-specific T cell increases the expression of activation markers such as CD137 (4-1BB), CD134 (OX40), or CD154 (CD40L) when stimulated with its specific cognate peptide/MHC complex. Peripheral blood mononuclear cells (PBMCs) are first treated in vitro with a peptide or peptide pool of interest to induce the expression of activation markers on antigen-reactive T cells. The PBMCs are then stained with a commercially available Phycoerythrin (PE)-conjugated antibody that recognizes the activation-induced marker of interest. Antigen-reactive T cells are targeted for enrichment using a PE-specific positive selection antibody cocktail that binds to PE and magnetic particles. Cells labeled with magnetic particles are then separated using a magnet.
2. Tetramer Staining Method
This method targets antigen-specific CD8+ T cells in a PBMC suspension with a PE-conjugated HLA class I-peptide tetramer. The tetramer-labeled CD8+ T cells are then targeted with a PE-specific positive selection antibody cocktail that binds PE and magnetic particles. Cells labeled with magnetic particles are then separated using a magnet.
Comparing the AIM and Tetramer Staining Methods
The AIM and tetramer staining methods are both valuable tools for isolating antigen-specific T cells, but they differ in their approaches. The AIM method requires in vitro stimulation of PBMCs to induce the expression of activation markers on antigen-reactive T cells. The tetramer staining method does not require in vitro stimulation or the induction of activation markers. Instead, it relies on the binding of HLA class I-peptide tetramers to matching T cell receptors (TCRs) to isolate the antigen-specific CD8+ T cells.
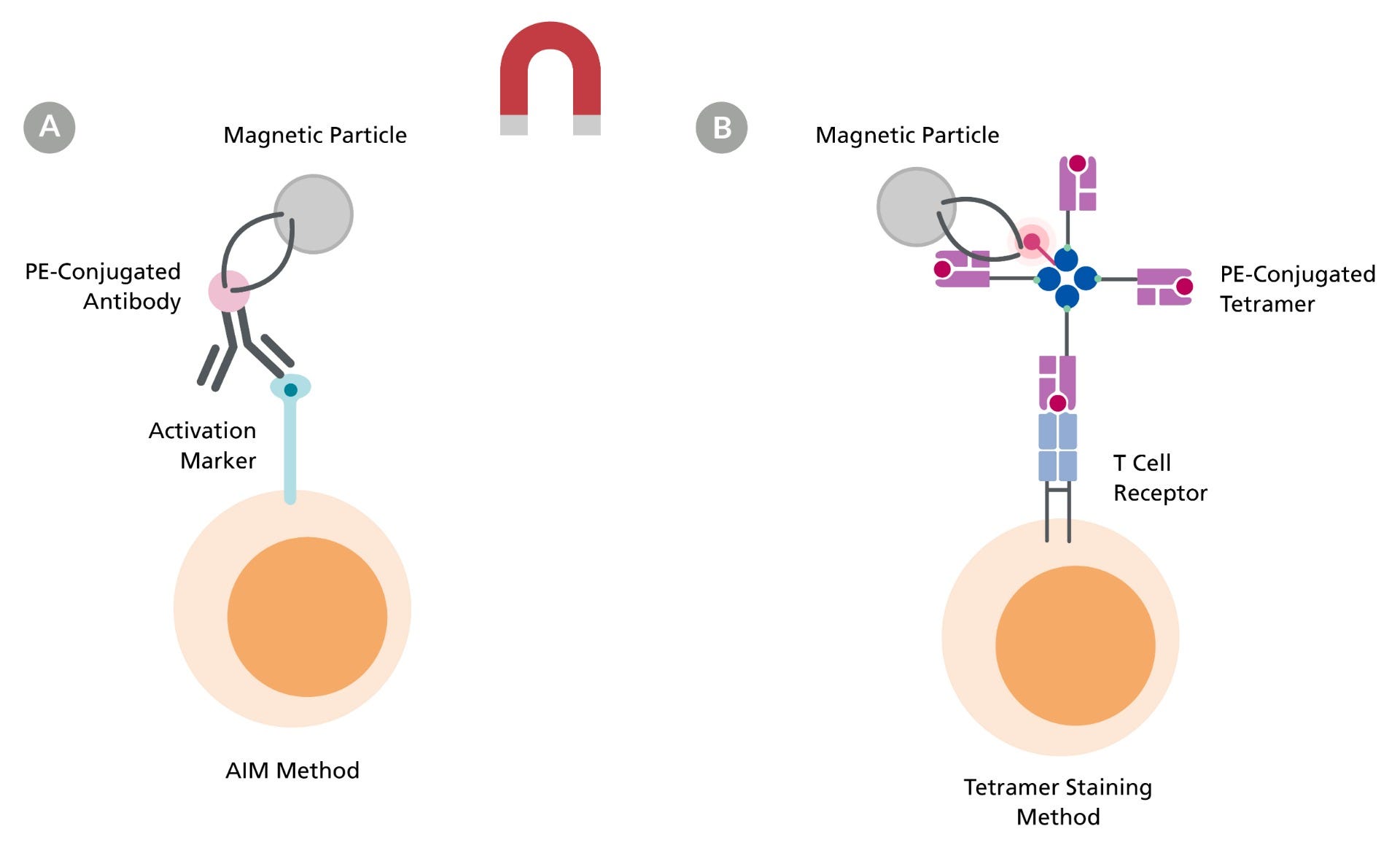
Figure 1. Isolation of Antigen-Specific T cells Using AIM or Tetramer Staining Methods
A. Detection of antigen-reactive T cells using PE-conjugated antibodies that bind to a T cell activation marker of interest. The desired cells are labeled with a positive isolation antibody cocktail and magnetic particles before being separated using a magnet. B. Targeting antigen-specific T cells with PE-conjugated HLA class I-peptide tetramer. Desired cells are labeled with a positive isolation antibody cocktail and magnetic particles before being separated using a magnet.
The following protocols describe how to isolate antigen-specific T cells using the AIM and tetramer strategies with EasySep™ Release Human PE Positive Selection Kit (Catalog #17654) or EasySep™ Human PE Positive Selection Kit II (Catalog #17664). EasySep™ Release Human PE Positive Selection Kit is recommended if the positive selection marker is a PE-conjugated HLA class I-peptide tetramer, a PE-conjugated anti-human CD137 antibody, or when particle-free positively selected cells are required for downstream applications. EasySep™ Human PE Positive Selection Kit II can be used for the enrichment of T cells labeled with PE-conjugated HLA class I-peptide tetramers, or PE-conjugated antibodies that bind to CD134, CD137, or CD154. Both protocols have undergone in-house testing and provide a reliable guide to support your antigen-specific T cell isolation experiment design. These protocols may be adjusted to fit your research needs.
For further assistance and information on other possible product choices to isolate antigen-specific T cells, please contact techsupport@stemcell.com.
Materials
Cell Sourcing
T cells are isolated from PBMCs, which can be sourced from different human primary cell sources,* including whole blood and leukopaks.
Cell Culture and Cell Isolation Materials
Cell Culture Media and T Cell Activation Reagents
- ImmunoCult™-XF T Cell Expansion Medium (Catalog #10981)
- CMV (pp65) Peptide Pool (Catalog #100-0668)
- Influenza (HLA Class I Control) Peptide Pool (Catalog #100-0672)
Antibodies for Indirect Positive Selection
- PE Mouse Anti-Human CD137 (BD Biosciences, Catalog #555956)
- iTAg Tetramer/PE – HLA-A*02:01 CMV pp65 (NLVPMVATV) (MBL International Corporation, Catalog #TB-0010-1)
Cell Isolation Materials
- EasySep™ Release Human PE Positive Selection Kit (Catalog #17654)
- EasySep™ Human PE Positive Selection Kit II (Catalog #17664)
- EasySep™ Buffer (Catalog #20144)
- EasySep™ Magnet (Catalog #18000)
Cultureware
- Costar® 6-Well Flat-Bottom Plate, Tissue Culture-Treated (Catalog #38015)
- Costar® 12-Well Flat-Bottom Plate, Tissue Culture-Treated (Catalog #200-0624)
- 5 mL (12 x 75 mm) polystyrene round-bottom tube (Catalog #38007)
*Certain products are only available in select territories. Please contact your sales representative or the Product & Scientific Support team at techsupport@stemcell.com for further information.
Protocols
Pre-Isolation Peptide Activation of Antigen-Specific T Cells with AIM Method
If using the AIM method, first perform the in vitro stimulation step with human PBMCs, prior to the antigen-specific T cell isolation. For this step, incubate the PBMCs with the viral peptide pools of choice for the recommended duration to induce expression of activation markers such as CD137 (4-1BB). The protocol below provides suggested steps to stimulate antigen-specific T cells with the CMV (pp65) Peptide Pool.
- Prepare a PBMC suspension at 1 x 107 cells/mL in ImmunoCult™-XF T Cell Expansion Medium for T cell expansion and seed the cells to either 6-well or 12-well tissue culture plates at a cell density of 5 x 106 cells/cm2.
- Add the viral peptide pool(s) at 1 μg/mL per peptide to the cell suspension to stimulate the T cells. Mix the cells and peptide(s) carefully and incubate at 37°C and 5% CO2 for the recommended time.
Note: Antibody stimulation conditions
Optimal time points differ for each antibody-induced marker, and must be optimized. As a starting point, activate cells for 16 - 24 hours for CD137, 24 - 48 hours for CD134, and 6 - 18 hours for CD154. - Harvest the cells after stimulation by gently pipetting up and down to ensure all cells are in suspension, then transfer to an appropriate tube from the tissue culture plates.
Optional: To improve PBMC recovery, wash each well with 1 - 2 mL of PBS and add the recovered cells to the harvested cells.
- Centrifuge the cells at 300 x g for 10 minutes (low brake) at room temperature. Discard the supernatant and resuspend the cells in EasySep™ Buffer at the recommended minimal volume for EasySep™ Release Human PE Positive Selection Kit or EasySep™ Human PE Positive Selection Kit II (indicated in the EasySep™ Release Human PE Positive Selection Kit Protocol step 1 below).
- Count the cells using 3% Acetic Acid with Methylene Blue (see How to Count Cells with a Hemocytometer). Trypan blue may also be used to assess cell viability.
- Dilute the PBMCs to 1 x 108 cells/mL with EasySep™ Buffer, or use at the recommended minimum volume.
EasySep™ Release Human PE Positive Selection Kit Protocol
This protocol section describes how to isolate antigen-specific T cells from PBMCs using EasySep™ Release Human PE Positive Selection Kit for tetramer or CD137 (AIM method) staining.
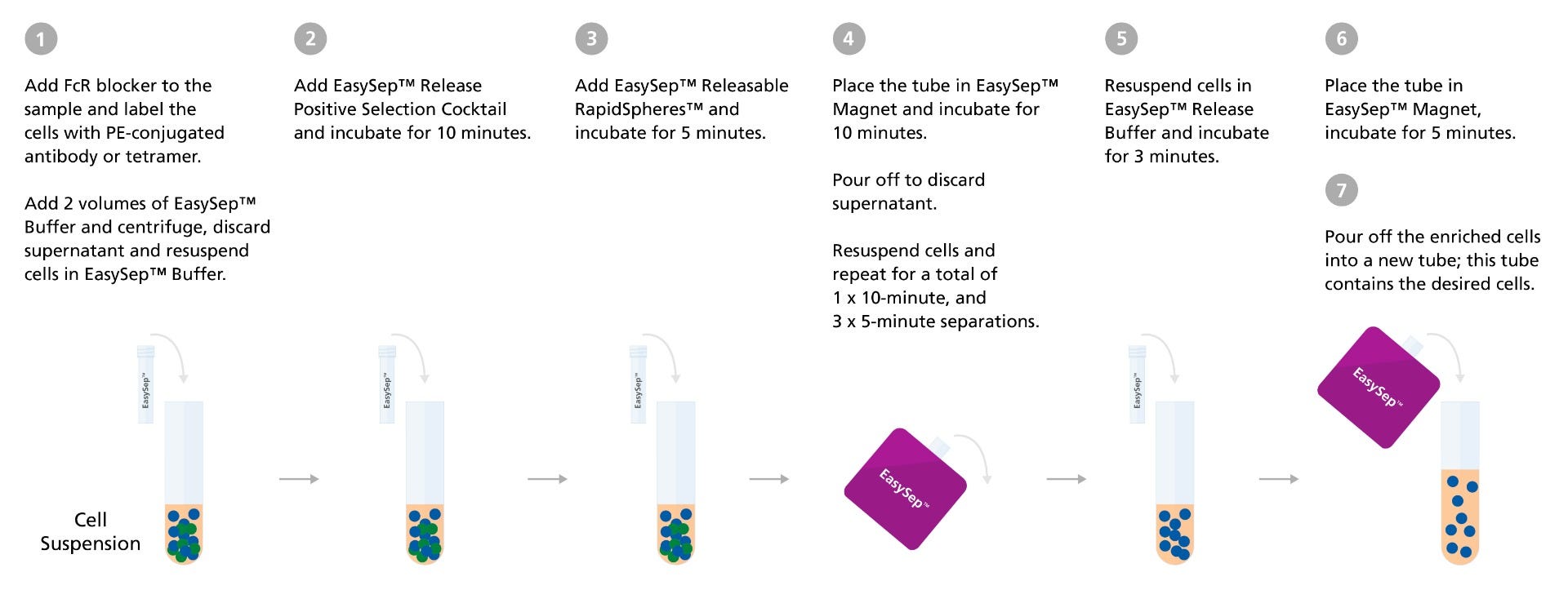
Figure 2. EasySep™ Release Human Positive Selection Kit Protocol Diagram
A. Detection of antigen-reactive T cells using PE-conjugated antibodies that bind to a T cell activation marker of interest. The desired cells are labeled with a positive isolation antibody cocktail and magnetic particles before being separated using a magnet. B. Targeting antigen-specific T cells with PE-conjugated HLA class I-peptide tetramer. Desired cells are labeled with a positive isolation antibody cocktail and magnetic particles before being separated using a magnet.
- Prepare a PBMC suspension (containing antigen-specific T cell population) in EasySep™ Buffer at 1 x 108 cells/mL (maximum volume of 2 mL) in a 5 mL (12 x 75 mm) polystyrene round-bottom tube. If starting with fewer than 5 x 107 cells, resuspend cells in 0.5 mL.
- Add EasySep™ Anti-Human CD32 (Fc gamma RII) Blocker (Component #18520) to the cell suspension at 200 μL/mL of sample. Mix and incubate at room temperature for 5 minutes.
- Label the cells of interest with desired antibodies:
- For the AIM method, label the T cells with PE-conjugated antibodies against the target activation marker(s) of interest; e.g. if using CD137 as the selection marker, add PE-conjugated anti-human CD137 antibody (clone 4B4-1). The primary antibody concentration needs to be optimized for each PE-conjugated reagent; however, 1 μg/mL of PE-conjugated antibody is recommended as a starting point. Incubate for 10 minutes at room temperature, protected from light.
- For the tetramer staining method, add PE-conjugated tetramer such as the CMV pp65-specific PE-conjugated tetramer (iTAg Tetramer/PE – HLA-A*02:01 CMV pp65 (NLVPMVATV)). The tetramer concentration needs to be optimized for each PE-conjugated tetramer; however, 10 μL/mL of PE-conjugated tetramer is recommended as a starting point. Incubate for 30 minutes at room temperature, protected from light.
- Add 2 volumes of EasySep™ Buffer and centrifuge the cells at 300 x g for 10 minutes (low brake).
- Carefully aspirate and discard supernatant, and resuspend the cells with EasySep™ Buffer at the same volume as in step 1. Once resuspended, return the sample to the tube used for step 1.
Note: If needed, the wash step can be performed in a larger tube.
- Add EasySep™ Release PE Positive Selection Cocktail (Component #17654C) to the sample at 100 μL/mL. Mix and incubate for 10 minutes at room temperature.
- Vortex EasySep™ Releasable Rapidspheres™ (Component #50201) for 30 seconds.
Note: Particles should appear evenly dispersed.
- Add EasySep™ Releasable Rapidspheres™ to the sample at 75 - 100 μL/mL. Mix and incubate for 5 minutes at room temperature.
Note: Adjust EasySep™ Releasable Rapidspheres™ volume depending on whether high purity or high recovery is the priority; recommended volume is 75 μL/mL for purity and 100 μL/mL for recovery.
- Add EasySep™ Buffer to top up the sample to 2.5 mL. Mix by gently pipetting up and down 2 - 3 times.
- For the magnetic separations, place the tube without a lid into the purple EasySep™ Magnet and incubate for 10 minutes (first magnet incubation step).
Note: Cells are subjected to four magnet incubation steps, the first for 10 minutes.
- Pick up the magnet and, in one continuous motion, invert the magnet and tube, pouring off the supernatant. Remove the tube from the magnet; this tube contains the desired positively selected cells.
- Resuspend cells in 2.5 mL of EasySep™ Buffer.
- Place the tube (without lid) into the purple EasySep™ Magnet and incubate for 5 minutes (second magnet incubation step).
- Pick up the magnet, and, in one continuous motion, invert the magnet and tube, pouring off the supernatant. Remove the tube from the magnet; this tube contains the desired positively selected cells.
- Resuspend cells in 2.5 mL of EasySep™ Buffer.
- Repeat Steps 13 and 14 twice for a third and fourth separation step.
Optional: Reduce magnetic steps for higher recovery (but reduced purity) or when the start frequency of the desired cells is > 5%.
- Dilute EasySep™ Release Buffer (Component #20165) 1 in 40 with EasySep™ Buffer.
Note: EasySep™ Release Buffer (1X) must be prepared on the day of use.
- Add EasySep™ Release Buffer (1X) to top up the positively selected cell sample to 2.5 mL. Mix by gently pipetting up and down 2 - 3 times, and incubate for 3 minutes.
- Place the tube (without lid) into the magnet and incubate for 5 minutes. This step will remove the magnetic particles.
- Pick up the magnet, and in one continuous motion, invert the magnet and tube, pouring the enriched cell suspension into a new tube.
- Isolated cells (in the new tube) are now ready for use in downstream applications.
Optional: Centrifuge the cells at 300 x g for 10 minutes (low brake) to concentrate the isolated cells. Decant the supernatant and resuspend the isolated cells in the desired medium.
EasySep™ Human PE Positive Selection Kit II Protocol
This protocol section describes how to isolate antigen-specific T cells from PBMCs using EasySep™ Human PE Positive Selection Kit II for tetramer, CD134, CD137, or CD154 stainings.
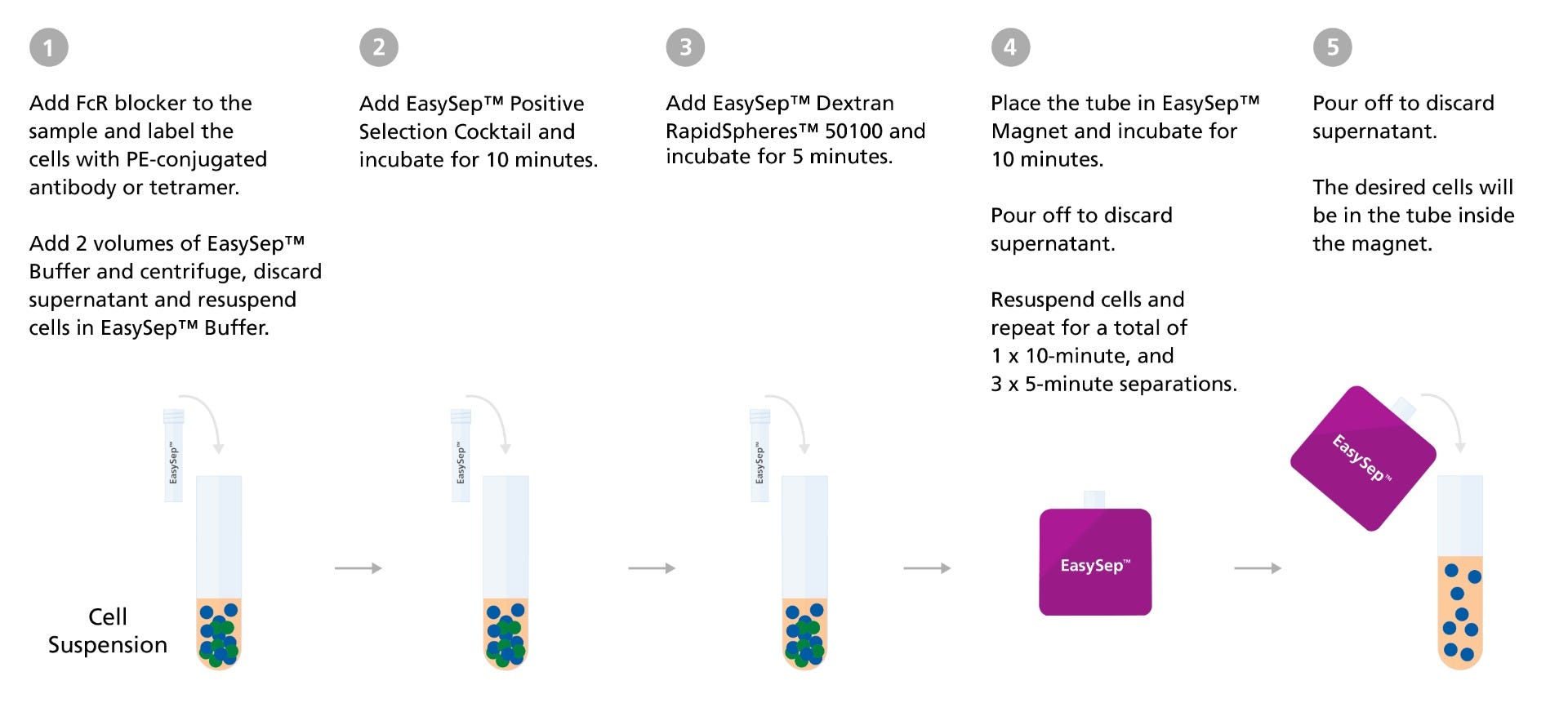
Figure 3. EasySep™ Human Positive Selection Kit II Protocol Diagram
Prior to separation, cells are treated with an Fc receptor (FcR) blocker. Cells are then labeled with PE-conjugated primary antibodies or PE-tetramer at room temperature, followed by the addition of the EasySep™ Positive Selection reagents. Following magnetic separation, isolated cells are resuspended in the desired medium and are ready for use in downstream applications.
- Prepare a PBMC suspension (containing antigen-specific T cell population) in EasySep™ Buffer at 1 x 108 cells/mL (maximum volume of 2 mL) in a 5 mL (12 x 75 mm) polystyrene round-bottom tube. If starting with fewer than 2 x 107 cells, the minimum PBMC cell suspension volume is 0.2 mL at 1 x 108 cells/mL.
- Add EasySep™ Anti-Human CD32 (Fc gamma RII) Blocker (Component #18520) to the cell suspension at 200 μL/mL of sample. Mix and incubate at room temperature for 5 minutes.
- Label cells of interest with desired antibodies:
- For the AIM method, label the T cells with PE-conjugated antibodies against the target activation marker(s) of interest; e.g. if using CD137 as the selection marker, add PE-conjugated anti-human CD137 antibody (clone 4B4-1). The primary antibody concentration needs to be optimized for each PE-conjugated reagent; however, 1 μg/mL of PE-conjugated antibody is recommended as a starting point. Incubate for 10 minutes at room temperature, protected from light.
- For the tetramer staining method, add PE-conjugated tetramer such as the CMV pp65-specific PE-conjugated tetramer (iTAg Tetramer/PE – HLA-A*02:01 CMV pp65 (NLVPMVATV)). The primary tetramer concentration needs to be optimized for each PE-conjugated tetramer; however, 10 μL/mL of PE-conjugated tetramer is recommended as a starting point. Incubate for 30 minutes at room temperature, protected from light.
- Add 2 volumes of EasySep™ Buffer and centrifuge the cells at 300 x g for 10 minutes (low brake).
- Carefully aspirate and discard supernatant, and resuspend the cells with EasySep™ Buffer at the same volume as in step 1. Once resuspended, return the sample to the tube used for step 1.
Note: If needed, the wash step can be performed in a larger tube.
- Add EasySep™ PE Positive Selection Cocktail (Component #18151) to sample at 100 μL/mL. Mix and incubate for 10 minutes at room temperature.
- Vortex RapidSpheres™ for 30 seconds.
Note: Particles should appear evenly dispersed.
- Add EasySep™ Dextran Rapidspheres™ (Component # 50100) to sample at 12.5 - 50 μL/mL. Mix and incubate for 5 minutes at room temperature.
Note: Adjust EasySep™ Dextran Rapidspheres™ volume depending on whether high purity or high recovery is the priority; recommended volume is 12.5 μL/mL for purity and 50 μL/mL for recovery.
- Add EasySep™ Buffer to top up the sample to 2.5 mL. Mix by gently pipetting up and down 2 - 3 times.
- For the magnetic separations, place the tube without a lid into the purple EasySep™ Magnet and incubate for 10 minutes (first magnet incubation step).
- Pick up the magnet and, in one continuous motion, invert the magnet and tube, pouring off the supernatant. Remove the tube from the magnet; this tube contains the desired positively selected cells.
- Resuspend cells in 2.5 mL of EasySep™ Buffer.
- Place the tube (without lid) into the purple EasySep™ Magnet and incubate for 5 minutes (second magnet incubation step).
- Pick up the magnet and, in one continuous motion, invert the magnet and tube, pouring off the supernatant. Remove the tube from the magnet; this tube contains the desired positively selected cells.
- Resuspend cells in 2.5 mL of EasySep™ Buffer.
- Repeat Steps 13 and 14 twice for a third and fourth separation step.
Optional: Reduce magnetic steps for higher recovery (but reduced purity) or use when the start frequency of the desired cells is > 5%.
- Resuspend cells in desired medium. Be sure to collect cells from the sides of the tube. Isolated cells are now ready for use in downstream applications.
Data Figures
The protocols above describe the isolation of antigen-specific T cells with EasySep™ Release Human PE Positive Isolation Kit and EasySep™ Human PE Positive Isolation Kit II. The flow cytometry data figures below show the antigen-specific T cell enrichment achieved by these two EasySep™ kits, illustrating the effectiveness of each method.
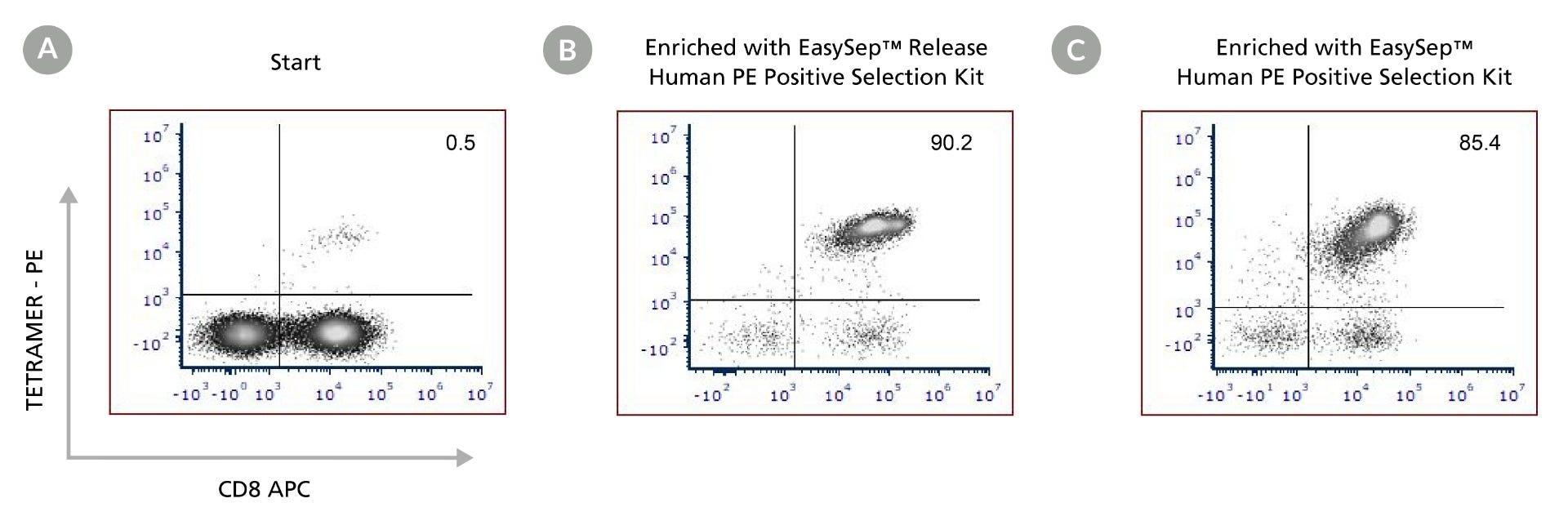
Figure 4. Enrichment of Tetramer-Labeled CD8+ T Cells Using EasySep™ Human PE Positive Selection Kits
PBMCs from fresh or frozen donors were labeled with PE-conjugated iTag HLA-A*02:01 CMV pp65 (NLVPMVAVT) class I tetramer. Antigen-specific CD8+ T cells were enriched using either EasySep™ Release Human PE Positive Selection Kit (b) or with EasySep™ Human PE Positive Selection Kit II (c). Samples were stained with antibodies to human CD3 and CD8 and the viability dye Draq7™. Starting with tetramer-labeled PBMCs, the purity at the start (a) was 0.5%, and the purities of the final isolated cells (b and c) were 90.2% and 85.4% using EasySep™ Release PE Human Positive Selection Kit, and EasySep™ Human PE Positive Selection Kit II, respectively. Data is representative of two independent PBMC donors.
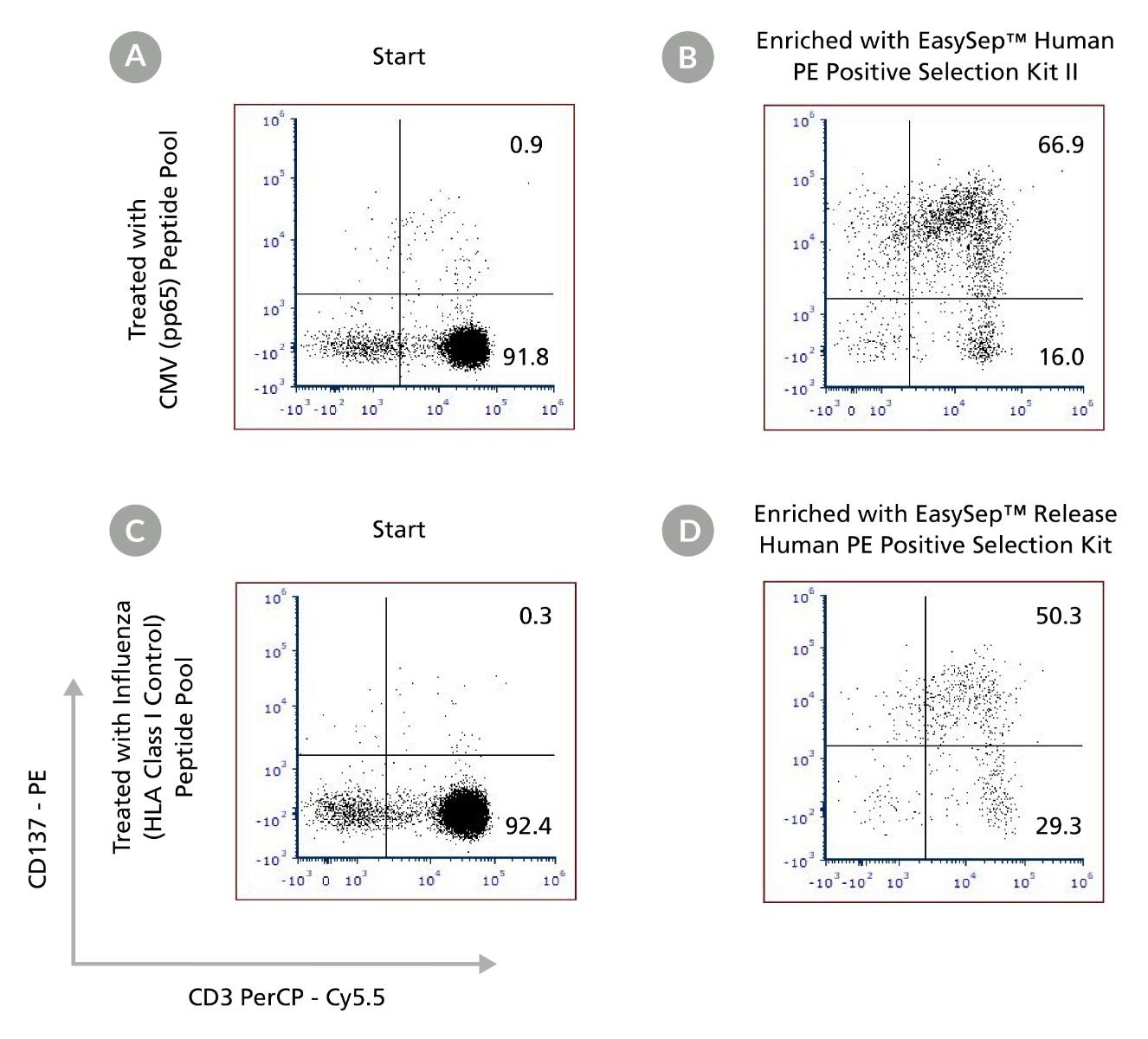
Figure 5. Enrichment of CD137+ T Cells Activated by Viral Peptide Pools Using EasySep™ Human PE Positive Selection Kits
PBMCs were cultured overnight with ImmunoCult™-XF for T cell Expansion Medium in the presence of either CMV pp65 peptide pool (a and b) or Influenza (HLA Class I Control) peptide pool (c and d). Cells were then labeled with PE-conjugated anti-human CD137 antibody (clone 4B4-1). Antigen-induced CD137+ T cells were enriched using either EasySep™ Human PE Positive Selection Kit II (b) or EasySep™ Release Human PE Positive Selection Kit (d). Samples were stained with antibodies to human CD3 and the viability dye Draq7™. Starting with PE-labeled PBMCs, the purities of the start (a and c) and enriched samples (b and d) were ≤ 1% and ≥ 50% viable T cells, respectively. Data is representative of two independent PBMC donors.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration