Dr. Wardiya Afshar Saber describes her work examining synaptic activity in Alzheimer’s disease using optogenetics and stem cell technology
A New Way to Study Neural Network Connectivity

Wardiya Afshar Saber is a Doctoral Researcher at the School of Medicine, University of St. Andrews. Previously, she worked in Professor Clifford Woolf’s laboratory at the Harvard Medical School and Dr. Cédric Ghevaert’s laboratory at the University of Cambridge. Her current work focuses on the development of an all-optical assay to investigate synaptic activity in Alzheimer’s disease using optogenetics and stem cell technology.
Additionally, we observed a significant lower mean global connectivity in the Neurobasal® condition as compared to BrainPhys™ medium which also agrees with previous published findings (Bardy et al., 2015) showing that BrainPhys™ supports optimal action potentials and synaptic activity, while Neurobasal medium reduces synaptic communication and action potential firing.
Saber WA et al. (2018) All-Optical Assay to Study Biological Neural Networks. Frontiers in Neuroscience. 12:451.
Wardiya's Story
What made you choose scientific research as a career path?
I am fascinated by science, medicine, and research. There is always something to search, create, discover. I also believe that science should be viewed from a broad and multidisciplinary perspective. Working in collaborative and interdisciplinary environments stimulates my scientific curiosity and ability to generate original ideas.
Who/What inspires you?
I am inspired by creativity, novelty, and cutting-edge science. I was lucky to have mentors all around the world who inspired me with their passion for research and their contribution to major scientific advances. I hope my research will also have palpable benefits for our society.
What is your current research focus?
I am interested in understanding the mechanisms underlying memory, mapping the brain connectivity, and how this is affected in neurodegenerative diseases. I am fascinated by regenerative medicine, stem cells, and optogenetics.
The Paper: All-Optical Assay to Study Biological Neural Networks1
What is the significance of using optogenetics to study neural networks?
Using optogenetics as an approach to study neural networks allows repetitive interrogation of the same cells over time in a contact-free manner to preserve the network integrity. By preserving the network integrity, we can perform experiments over many days making it a potential tool for studies on long-term processes such as network formation or neurodegeneration. Additionally, the use of optogenetics allows [us] to target various neuronal cell types in co-cultures by using specific promoters.
Can you elaborate on your novel all-optical assay?
I have created a plasmid called OptoCaMP that allows simultaneous optical stimulation and recording from large populations of neurons with a single-cell readout. It is a combination of a Channelrhodopsin variant (CheRiff) and a genetically encoded calcium indicator (jRCaMP1b). This plasmid can be used with an all-optical assay, allowing the investigation of the spread of excitation through an interconnected network to investigate changes in neural network connectivity.
How do you see your assay being used? How about high-throughput potential?
This assay brings the promise to enable the study of more complex pharmacological conditions or disease models affecting connectivity. For example, our system could be easily integrated with hardware platforms used for quantitative in vitro high-throughput screening and could potentially be adapted to multi-well plates. This capability could be achieved using automated liquid handlers and other currently available automated platforms to investigate how diseased neural networks communicate and respond to potential therapeutic agents.
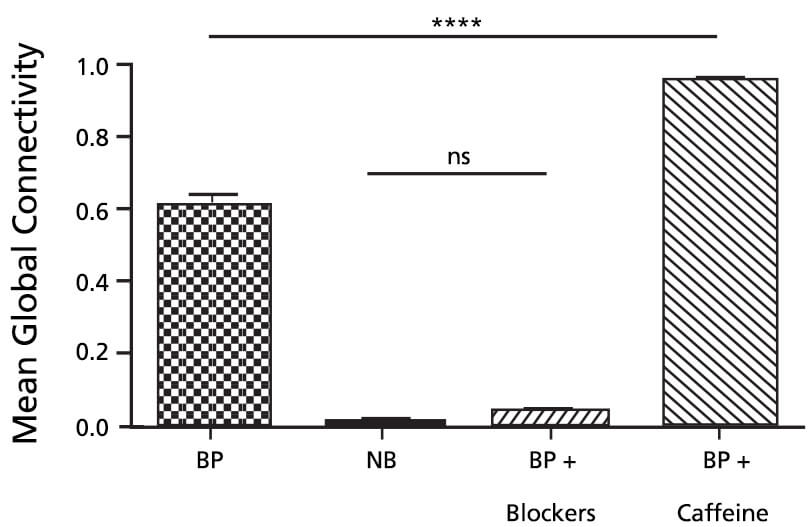
Figure 1. Mean Global Connectivity in BrainPhys™ Complete Medium (BP), Complete Neurobasal® Medium (NB), Synaptic Blockers (BP + blockers) and Caffeine (BP + caffeine) Conditions.
n = 3 independent cell culture experiments for each condition; one-way ANOVA, F(3,8) = 854, P<0.0001; followed by Tukey’s multiple comparisons test: ns=non-significant, ∗∗∗∗P < 0.0001. Reproduced from Figure 4D from Afshar Saber W, Gasparoli FM, Dirks MG, Gunn-Moore FJ and Antkowiak M (2018) All-Optical Assay to Study Biological Neural Networks. Front. Neurosci. 12:451. doi: 10.3389/fnins.2018.00451. Material is licensed under the CC BY 4.0. Copyright © 2018 Afshar Saber, Gasparoli, Dirks, Gunn-Moore and Antkowiak.
How Wardiya Used BrainPhys™
In your paper, you performed a proof-of-concept neural network connectivity experiment (Figure 1) to verify your assay, can you describe it?
Functional studies of biological neural networks are fundamental to understand brain activity, to investigate how these networks are altered in brain disorders, and to help develop new treatments.2 To explore heterogeneous neural networks, it is often critical to understand the activity of multiple neurons and how they communicate with each other. To capture the connectivity, it is essential to have a tool [that is] potentially applicable to both 2D and 3D neuronal culture systems, [and] that enables the simultaneous stimulation and recording from a large population of neurons with single-cell readout. The use of OptoCaMP along with the stimulation of a sub-section of a neural network enables quantification of a network’s connectivity. Using this technique in various conditions, we observed that the addition of caffeine and synaptic blockers, respectively resulted in a significant increase and decrease of the mean global connectivity as compared to the BrainPhys™ medium control condition. These results agree with the documented increase of the neuronal excitability with caffeine3,4 and depression with the addition of synaptic blockers.5 Additionally, we observed a significant lower mean global connectivity (Figure 1) in the Neurobasal® condition as compared to BrainPhys™ medium which also agrees with previous published findings showing that BrainPhys™ supports optimal action potentials and synaptic activity, while Neurobasal medium reduces sYnaptic communication and action potential firing.6
How and why did you decide to use BrainPhys™ in your experiment?
I decided to use BrainPhys™ in my experiments after reading the publication by Bardy et al.6 BrainPhys™ has been optimized to support both excitatory and inhibitory synaptic function and to mimic as close as possible in vivo brain conditions. What interested me was the fact that BrainPhys™ basal medium was more efficient to support physiological action potential firing and synaptic activity.
Should neuroscientists be concerned with whether they are using a basal medium that inhibits neural connectivity in cultures?
I believe the choice of medium varies with the application. My research focuses on neural network function and connectivity therefore, the medium I use should ideally support basic synaptic functions and activity of neurons. As demonstrated in my publication, BrainPhys™ supplemented with NeuroCult™ SM1 was adequate for this application compared to other basal medium that reduces synaptic communication.1
References
- Saber WA et al. (2018) All-Optical Assay to Study Biological Neural Networks. Frontiers in Neuroscience. 12:451.
- Alivisatos et al. (2012) The brain activity map project and the challenge of functional connectomics. Neuron. 2012 74(6):970-4.
- Yanovsky Y et al. (2006) Ca2+ release-dependent hyperpolarizations modulate the firing pattern of juvenile GABA neurons in mouse substantia nigra pars reticulata in vitro. J. Physiol. 577, 879–90.
- van Aerde K et al. (2015) Cell type-specific effects of adenosine on cortical neurons. Cereb. Cortex 25, 772–87.
- Lin et al. (2002) Development of excitatory synapses in cultured neurons dissociated from the cortices of rat embryos and rat pups at birth. J. Neurosci. Res. 67, 484–93.
- Bardy C et al. (2015) Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc Natl Acad Sci. 112 (20) E2725-34.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration


