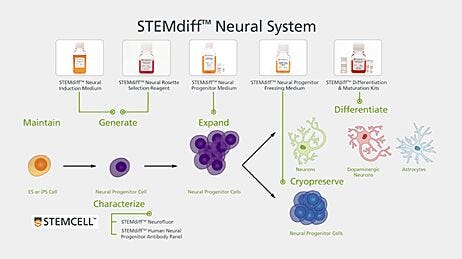
Neurological Disease
Modeling Genetic Epilepsies with Induced Pluripotent Stem Cells
Human induced pluripotent stem (iPS) cell models of epilepsy are becoming a revolutionary platform for mechanistic studies and drug discovery. The skyrocketing pace of epilepsy gene discovery is vastly outstripping the development of in vivo animal models. Currently, antiepileptic drug prescribing to patients with specific genetic epilepsies is based on small-scale clinical trials and empiricism; however, rapid production of patient-derived iPS cell models will allow for precision therapy. In this webinar presented by Dr. Andrew Tidball, he discusses current and potential uses of iPS cells for modeling epilepsies, how to develop different iPS cell-based model systems, as well as the key advantages and drawbacks of using these model systems.

Jack Parent Lab, University of Michigan
- Epilepsy genetics and the use of iPS cells in seizure-related disease research
- Generation and gene-editing of iPS cells
- Neuronal differentiation methods and their advantages and drawbacks
- Outcome assays, including cell autonomous and non-cell autonomous methods
Jack Parent Lab, University of Michigan
Dr. Andrew Tidball obtained his PhD at Vanderbilt University in the lab of Dr. Aaron Bowman, where he used iPS cells to study the potential role of environmental metal ion exposure in Huntington’s and Parkinson’s diseases. His overall interests lie in using human iPS cells to model early life neurological disorders. Investigating these diseases provides insights into both potential therapies and important biological functions in the developing brain.
Dr. Tidball’s current work in the Parent lab involves modeling severe genetic epilepsies in neurons from patient-derived iPS cells. He is interested in drug discovery, particularly in the context of identifying gene-specific therapies optimized for genetic subgroups of epilepsy patients. Dr. Tidball uses gene-editing techniques to generate isogenic controls and “virtual” patient iPS cell lines that he differentiates into neurons for functional analysis.
From Pluripotent Stem Cells to Neurons and Astrocytes - Modeling Human Neurological Disease
Mature neuronal and glial cells derived from human pluripotent stem cells (hPSCs) have emerged as a physiologically relevant model for the study of neurological development and disease. The derivation of these cells has already surpassed the “proof of concept” stage and is now changing the way researchers approach disease modeling and drug development. In this webinar, learn how to efficiently generate forebrain-type neurons, dopaminergic neurons and astrocytes from normal and diseased iPS cells. Prof. Xianmin Zeng describes how the neurons and astrocytes generated have been successfully used in toxicological studies and mechanism-of-action studies in Parkinson’s disease.

Buck Institute
- Strategies for generating astrocytes, dopaminergic neurons and forebrain-type neurons from multiple ES and iPS cell lines
- Biochemical and functional characterization of hPSC-derived neuronal and glial cells
- Applications of hPSC-derived neuronal and glial cells in neurotoxicological and neuroprotective screens
- Applications of hPSC-derived cells for neurological disease modeling and potential cell therapy
Harvard University
Prof. Xianmin Zeng obtained her PhD in Molecular Biology at the Technical University of Denmark and continued onto postdoctoral training at the National Institutes of Health, where she began her research on human embryonic stem cells. Currently, she is an Associate Professor at the Buck Institute for Research on Aging, studying neural developments in humans with a focus on Parkinson’s disease. Prof. Xianmin has developed a method to induce embryonic and induced pluripotent stem cells to dopaminergic neurons, the cells depleted in Parkinson’s disease. Prof. Xianmin is also the founder of the biotechnology company XCell Science.
Neuronal Phenotyping of an iPS Cell Model of Rett Syndrome
Induced pluripotent stem (iPS) cell-based models hold great potential for the study of human neurological disease and disorders. Patient-derived iPS cells are increasingly being used to generate neurons, with the goal of recapitulating and studying disease phenotype in vitro. Dr. Ross describes how patient-specific iPS cell-derived neurons can be functionally characterized and used to identify neurodevelopmental disorder-associated neuronal phenotypes.

The Hospital for Sick Children, Toronto
- Overview of disease modeling with iPS cells
- Establishment of an iPS cell-based model of Rett syndrome using human cells
- Differentiation of iPS cells to cortical neurons
- Methods for functional characterization of iPS cell-derived neurons
- Identification of Rett syndrome-associated neuronal phenotypes
The Hospital for Sick Children, Toronto
Dr. P. Joel Ross obtained his PhD from the University of Ottawa. He previously held a Research Associate position with Dr. James Ellis’ laboratory at the Hospital for Sick Children, where he used iPS cells to model Rett syndrome and autism spectrum disorder. Dr. Ross is currently an Assistant Professor in the Department of Biology at the University of Prince Edward Island.
The Road to Functional Human Neuronal Circuits in Vitro
Active neurons are a vital part of a functioning brain and an essential component of in vitro neurological models. Dr. Cedric Bardy talks about why and how he invented BrainPhys™, a new neuronal basal medium that better supports neuronal function in vitro, described in C Bardy et al. Proc Natl Acad Sci USA, 2015. Dr. Bardy describes how BrainPhys™ increases the physiological relevance of primary and hPSC-derived neuronal cultures in vitro.

Salk Institute for Biological Studies, California
- Overview of the media used for neuronal cultures
- Electrophysiological measurements of human neural cultures
- The advantages of neuronal media adapted for neurophysiological activity
Salk Institute for Biological Studies, California
Dr. Cedric Bardy obtained a PhD in Medicine from the University of Sydney in Australia, and pursued his postdoctoral training in Neuroscience at the Pasteur Institute in Paris and at the Salk Institute in California (Prof. Fred H. Gage’s laboratory). Dr. Bardy is an expert in human neural stem cell models and electrophysiology. His research efforts are focused on developing new stem cell biotechnologies and strategies to understand the effect of neurological disorders on brain cells.