Metabolic Disease
Investigating Metabolic Disease with Human Pluripotent Stem Cells
The ability of human pluripotent stem cells (hPSCs) to differentiate into the many specialized cell types of the human body holds great promise for the study and treatment of human disease. Genome editing of hPSCs can allow for more accurate modeling of human diseases by introducing disease-specific mutations or correcting genetic aberrations in patient-derived induced pluripotent stem cell lines. Advances in genetic tools, such as clustered regularly interspaced short palindromic repeat (CRISPR) technology, have improved the ease and efficiency of genome editing in hPSCs.
View this webinar presentation by Dr. Chad Cowan, who discussed his research efforts to understand metabolic disease using the CRISPR/Cas system in human pluripotent stem cells to create genetic disease models.

Harvard University, Cambridge, MA
- Culture conditions for expansion and cloning of hPSCs
- Design and use of TALENs or CRISPRs with hPSCs
- Applications of modified hPSCs in modeling metabolic disease
- Differentiation of hepatocyte-like cells
- Differentiation and chemical conversion of white to brown adipocytes
Harvard University
Dr. Chad Cowan obtained his PhD from the University of Texas Southwestern at Dallas and continued on to complete a postdoctoral fellowship in the lab of Dr. Douglas Melton at Harvard University. Currently, he is an Associate Professor at Harvard University in the Department of Stem Cell and Regenerative Biology and Massachusetts General Hospital, with appointments in the Center for Regenerative Medicine, Cardiovascular Research Center, and Center for Human Genetics Research. He is also an Associate Member of the Broad Institute and a Principal Faculty member of the Harvard Stem Cell Institute, where he directs the Diabetes Disease Program and the iPS Cell Core Facility. Chad uses genome editing to introduce disease-associated gene mutations into hPSCs and differentiates these cells into relevant tissue types to develop ex vivo disease models and for genetic and drug screens.
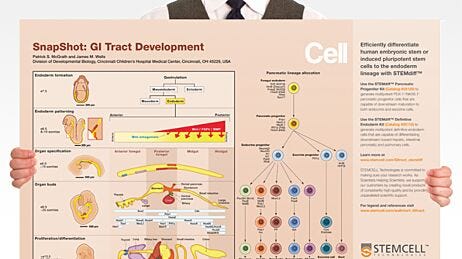
Understanding Pancreatic Development and Diabetes Using Patient-Specific iPS Cells
Pancreatic progenitor cells derived from human embryonic stem or induced pluripotent stem cells hold tremendous potential for modeling pancreatic development and disease, as well as for regenerative therapies addressing degenerative conditions like diabetes. The ability to direct differentiation of pancreatic progenitor cells to specific endocrine cell types has led researchers to propose human pluripotent stem cells as an unlimited source of insulin-producing beta cells for studying and treating diabetes.
View this webinar presentation by Dr. Ray Dunn and Dr. Jamie Trott, who discussed their research efforts to generate pancreatic progenitors and beta cells from diabetic patients from around the world.

A*STAR Institute for Medical Biology (IMB)

A*STAR Institute of Medical Biology (IMB)
- Generation of patient-specific pluripotent stem cells
- Pancreatic progenitor differentiation and expansion
- Beta cell differentiation
A*STAR Institute of Medical Biology (IMB)
Dr. Ray Dunn is a Principal Investigator at the A*STAR Institute for Medical Biology (IMB), Singapore. He also holds an Adjunct Assistant Professor position at the Lee Kong Chian School of Medicine at Nanyang Technological University (NTU) and the NTU School of Biological Sciences. Prior to joining IMB, Dr. Dunn was a Research Scientist and later a Program Manager in the Diabetes Group of the Singapore-based small biotech company ES Cell International. Dr. Dunn obtained his PhD in Cell Biology in 1999 from Vanderbilt University under the supervision of Dr. Brigid Hogan. He completed a postdoctoral fellowship in the lab of Dr. Elizabeth Robertson at Harvard University.
A*STAR Institute of Medical Biology (IMB)
Dr. Jamie Trott is a postdoctoral researcher in the lab of Dr. Ray Dunn at the A*STAR Institute of Medical Biology (IMB), Singapore. His research focuses on using patient-derived stem cells to develop in vitro models of pancreatic development and diabetic beta cell failure. Dr. Trott obtained his PhD in stem cell biology in the lab of Professor Alfonso Martinez-Arias at Cambridge University.
On Ray
What is your educational background?I grew up in rural, eastern North Carolina and attended Wake Forest University in Winston-Salem, where I double-majored in biology and French language and civilization, graduating with honors in May 1993. I then spent a little over a year abroad in Grenoble, France studying molecular and cellular biology at the Université Joseph Fourier. There, I was first introduced to developmental biology both in class and in the lab, working as an intern on a project investigating why chickens develop scales on their feet but feathers on their back. This basic question of patterning-how populations of initially equivalent cells later give rise to specific tissues in time and space-captivated me and led me to pursue a Ph.D. in developmental biology under the supervision of Dr. Brigid Hogan at Vanderbilt University Medical Center, Nashville, TN. During my Ph.D., I closely collaborated with Dr. Kirstie Lawson (then at Utrecht) and we showed that the secreted signaling molecule Bmp4 is absolutely required for formation of the mammalian germline-work that has now been cited more than 900 times. My rather unshakeable interest in embryonic development and lineage specification then carried me to Harvard University and the laboratory of Dr. Liz Robertson. There I investigated at the genetic level the contributions of Smad2 and Smad3, two intracellular effectors of the Nodal ligand, to germ layer specification during mouse gastrulation. It was during my postdoc that I began to home in on my now -favorite germ layer, the endoderm, which gives rise to the liver, lung and all-important pancreas.
Who is/are your scientific idols?
John Gurdon for his pioneering work on nuclear transfer and cloning; Rosa Beddington, an incomparable embryologist and molecular geneticist, who identified an extraembryonic signaling centre in the early mouse embryo responsible for the establishment of the anterior-posterior axis; James Thomson, of course, for the isolation of human embryonic stem cells in 1993; and Jennifer Doudna, Emmanuelle Charpentier and Feng Zhang for making gene editing a breeze-the advent of CRISPR/Cas9 has forever changed the world of research.
What's the focus of your current work?
My laboratory is fundamentally interested in elaborating the transcriptional networks governing human pancreatic development. We model in vitro rare forms of human pancreatic agenesis using gene-edited hESC or patient-derived human induced pluripotent cell (hiPSC) lines. Comparative approaches between wild-type and mutant cells have allowed us to identify the downstream transcriptional targets of key regulators of pancreatic development, such as PDX1. From these studies, we have selected several poorly characterized candidates for further validation, which we posit also function like PDX1 in the adult islet to maintain normoglycemia and are thus prone to dysregulation in diabetes. We are also pursuing the isolation of hESC-derived multipotent PDX1+ pancreatic progenitor cell lines that are both stable and scalable. Such an "intermediate" hESC-derived reagent, we argue, might overcome a number of the drawbacks of hESC-based directed differentiation and provides a novel and robust experimental platform for the downstream production of β cells for cell transplantation. Lastly, through a fortuitous collaboration within my home institute we are investigating rare forms of heritable diabetes and have focused our attention on genes potentially involved in the maintenance of β cell homeostasis.
On PSC Research
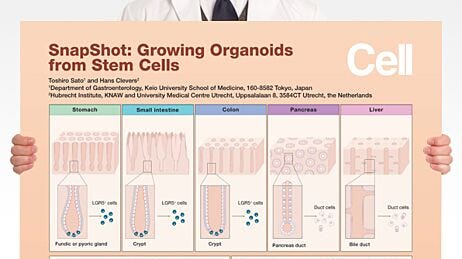
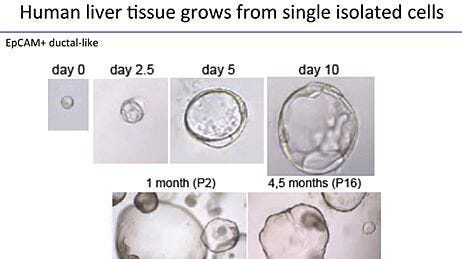
What do you consider to be the most important advance(s) in stem cell research over the past 5 years, and what advances do you hope the field will achieve in the next 5 years?Derivation of Metabolically Active Hepatocytes from Human Pluripotent Stem Cells
The ability of human pluripotent stem cells (hPSCs) to differentiate into the many specialized cell types of the human body holds great promise for the study and treatment of human disease. Genome editing of hPSCs can allow for more accurate modeling of human diseases by introducing disease-specific mutations or correcting genetic aberrations in patient-derived induced pluripotent stem cell lines. Advances in genetic tools, such as clustered regularly interspaced short palindromic repeat (CRISPR) technology, have improved the ease and efficiency of genome editing in hPSCs.
View this webinar presentation by Dr. Chad Cowan, who discussed his research efforts to understand metabolic disease using the CRISPR/Cas system in human pluripotent stem cells to create genetic disease models.

- Generation and characterization of definitive endoderm derived from hPSCs
- Methods for further differentiation of hPSC-derived definitive endoderm to metabolically active hepatocytes
- Applications of this research in drug development and cell therapy
Dr. David Hay is a Principal Investigator at the University of Edinburgh's MRC Centre for Regenerative Medicine. David has worked in the field of stem cell biology and differentiation over the last decade. His work has highlighted the important role that cell physiology and chemical biology plays in the generation of predictive and drug-inducible human hepatocytes from pluripotent stem cells. The impact of this work has led to a number of publications and regular appearances at high profile international conferences.
Most recently, David and his colleagues spun out a company from the University of Edinburgh, FibromEd, whose focus is to reduce the cost of human drug attrition.
On David
Tell us about yourself:I have a PhD from the University of St Andrews, and now I'm the Principal Investigator at the University of Edinburgh's MRC Centre for Regenerative Medicine. For the last decade, I've worked in the field of pluripotent stem cell biology and hepatocyte derivation.
My work is focused on human stem cell differentiation. We're interested in developing predictive human models and new medical devices. We have developed a robust stem cell differentiation process which generates high fidelity human hepatocytes. More recently, we have combined our approach with materials chemistry to deliver a more functional and stable in vitro system. This work has led to a University of Edinburgh spin-out company, FibromEd, which focuses on developing novel tools for research and industry.
On PSC Research
What do you consider to be the most important advance(s) in stem cell research over the past 5 years?Given our focus on cell based modelling, I would say the most important advances in our field are:
- a. Generation of human iPSCs from somatic cells permits selectable genotype.
- b. Generation of efficient differentiation processes and disease models that recapitulate human physiology.
- c. Demonstration that materials chemistry has an important role to play in the maintenance and differentiation of stem cells.
What advances do you hope the field will achieve in the next 5 years?