How to Establish, Expand, and Cryopreserve hPSC-Derived Liver Organoids
Liver organoids are increasingly being adopted as 3D, in vitro tissue modeling tools to study liver development, toxicity, and genetic and infectious diseases. Compared to traditionally used model systems, human liver organoids provide a more relevant model system that can recapitulate key hepatic functions and pathophysiology of diseases, as well as provide functional readouts, including CYP3A4 activity, albumin secretion, and urea production. Liver organoids derived from human pluripotent stem cells (hPSCs) also circumvent the problem of limited tissue availability while still capturing inter-individual features and remaining amenable to long-term culture, cryopreservation, and genetic manipulation.
Below, we describe a protocol for the generation and cryopreservation of hPSC-derived liver organoids from hepatocyte-like cells cultured using STEMdiff™ Hepatocyte Kit (Catalog #100-0520). Organoid cultures established using this protocol can be further expanded using HepatiCult™ Organoid Growth Medium (Human) (Catalog #100-0385) and differentiated with HepatiCult™ Organoid Differentiation Medium (Human) (Catalog #100-0383).
For complete instructions, use this document in coordination with the HepatiCult™ Product Information Sheet (Document #10000008301) and HepatiCult™ Technical Manual (Document #10000008300).
Materials
- hPSC-derived hepatic cells generated using the STEMdiff™ Hepatocyte Kit (Catalog #100-0520)
- HepatiCult™ Organoid Growth Medium (Human; Catalog #100-0385)
- 24-well tissue culture treated plate (e.g. Catalog #38017 OR Corning Catalog #3526)
- Corning® Matrigel® Growth Factor Reduced (GFR) Matrix (≥ 8 mg/mL protein; Corning Catalog #356231)
- D-PBS (Without Ca++ and Mg++) (e.g. Catalog #37350)
- 15 mL conical tubes (e.g. Catalog #100-0092)
Preparation
Thaw HepatiCult™ Organoid Growth Supplement overnight at 2 - 8°C. Mix thoroughly. Prepare 100 mL of complete HepatiCult™ Organoid Growth Medium (OGM) using sterile technique:
- 5 mL HepatiCult™ Organoid Growth Supplement
- 95 mL HepatiCult™ Organoid Basal Medium
- Mix thoroughly
Protocol
Establishment of hPSC-Derived Hepatic Organoids from Hepatic Monolayers:
The following protocol is for establishing hPSC-derived hepatic organoids in Corning® Matrigel® domes from cells generated using the STEMdiff™ Hepatocyte Kit at:
- Day 10, the end of stage 2 hepatic progenitor differentiation, or
- Day 21, the end of stage 3 hepatocyte-like cell differentiation
We suggest using a single well of hepatic progenitor cells or hepatocyte-like cells (HLCs) to seed 4 domes (i.e. using a split ratio of 1:4).
- Warm complete HepatiCult™ OGM to room temperature (15 - 25°C). Warm 24-well tissue culture-treated plates in a 37°C incubator for at least 1 hour. Thaw ~40 µL of Corning® Matrigel® on ice for each dome to be seeded.
Note: Keep Matrigel® on ice to prevent premature solidification. Pipette tips and other plasticware can be cooled at 2 - 8°C prior to working with Matrigel®.
- Without aspirating the spent medium, carefully scrape the cell layer from the bottom of the well using a 200 µL pipette tip. Transfer the entire contents of the well to a 15 mL conical tube. Pool up to 2 wells per tube.
- Gently pipette the total volume up and down 5 - 10 times using a 1 mL pipettor, taking care not to generate bubbles.
Note: This results in mechanical dissociation of the monolayer into smaller fragments of 30 - 100 µm. Assess fragment sizes using a light microscope; if most fragments are larger than 100 µm, triturate until they are ≤ 100 µm.
- Centrifuge the tube at 290 x g for 5 min. Carefully remove as much of the supernatant as possible without disturbing the pellet, leaving 5 - 10 µL in the tube. Place the tube on ice.
- Remove the warmed 24-well plate from the incubator and place in a biosafety cabinet.
- Process one tube/pellet at a time, as described below. Work quickly to ensure the Matrigel® does not solidify.
a. For every 1 well of hPSC-derived hepatic fragments collected in the tube, add 160 µL of thawed Matrigel® on top of the pellet. Without generating bubbles, gently mix the fragment-Matrigel® suspension by pipetting up and down 5 - 8 times, going to only the first stop of the pipettor.
Note: These volumes are sufficient to seed 4 domes, (i.e. a split ratio of 1:4).b. Using a pipettor with a 200 µL pipette tip, add 40 µL of the suspension to the center of one well of the 24-well plate to form a single dome. While dispensing, gradually move the pipette tip upward so that the fragments are evenly distributed throughout the dome. Dispense only to the first stop of the pipettor to avoid generating bubbles on top of the dome.
Note: The 8 wells in the center of a 24-well plate are the most suitable for domes since their surfaces are the most even. Wells at the edges of the plate are often slightly slanted, contributing to domes touching the wall of the well and flattening out. - Repeat step 6 for the remaining tubes/pellets.
- Place the lid on the culture plate. Carefully place the plate in an incubator at 37°C and 5% CO2 for 10 minutes to allow the domes to solidify.
- Remove the plate from the incubator and place it in the biosafety cabinet.
- Without disturbing the domes, carefully add 750 µL of room temperature complete HepatiCult™ OGM against the side of each well containing a dome. Do not pipette directly onto the domes.
- Add sterile D-PBS to any unused wells. Place the lid on the culture plate and place in a 37°C and 5% CO2 incubator.
- Perform a full-medium change every 2 - 3 days by carefully aspirating the medium from the well and adding 750 µL of fresh complete HepatiCult™ OGM at room temperature.
Note: If Matrigel® domes are loose, remove 500 µL of medium from the well and add 500 µL of fresh medium.Note: To avoid weekend medium changes, perform medium changes on Mondays, Wednesdays, and Fridays.
- Organoids will be ready to passage after approximately 1 week.
Note: Monitor the organoids daily. They should be passaged before the lumen darkens and the organoids collapse.
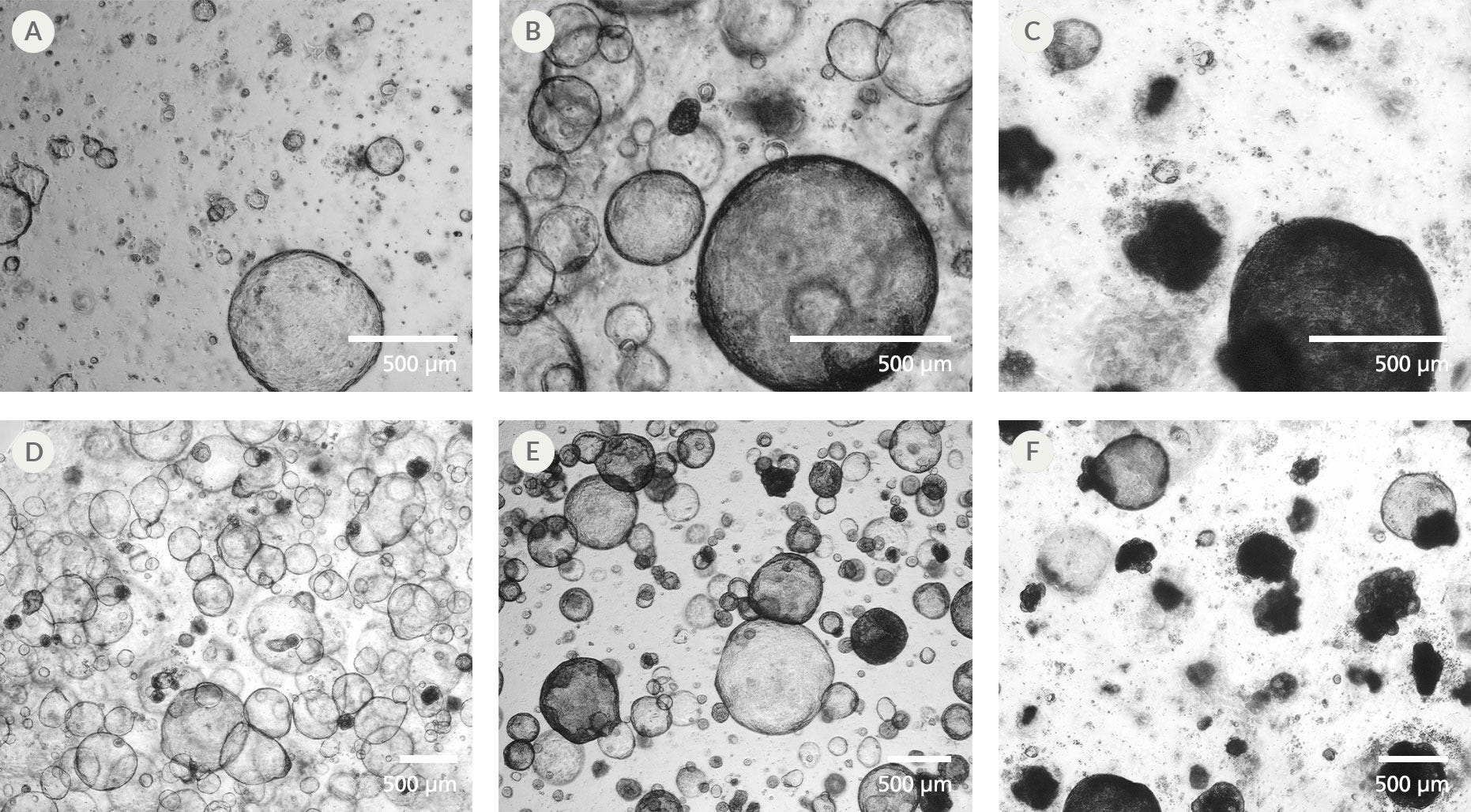
Figure 1. Assessing Optimal Times for Passage of hPSC-Derived Hepatic Organoid Cultures
hPSC-derived hepatic organoids should be passaged when the majority of organoids are greater than 100 µm in diameter with clear lumens (A, D). When several organoids in culture begin to exhibit dark lumens or collapse (B, E), cultures should be passaged immediately. Cultures are past the optimal passaging window when the majority of organoids have collapsed (C, F) and some debris is visible in the dome. Images shown are representative of organoids derived from hepatic progenitors. The same passaging guidelines should be followed for organoids derived from hepatocyte-like cells.
Passaging and Further Differentiating hPSC-Derived Hepatic Organoids:
Refer to sections 5.0 and 6.0 of the HepatiCult™ Technical Manual for complete hepatic organoid passaging and differentiation instructions.
Cryopreservation of hPSC-Derived Hepatic Organoids:
Refer to the Cryopreserving Hepatic Organoids Expanded in HepatiCult™ Organoid Growth Medium (Human) protocol.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration