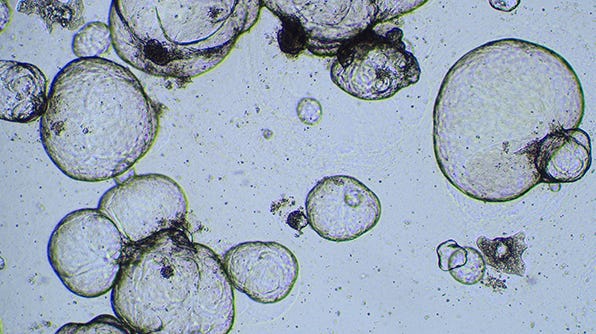
Cryopreserving Hepatic Organoids Expanded in HepatiCult™ Organoid Growth Medium (Human)
Cryopreserving hepatic organoids can be a key point in your experimental workflow as it offers a pausing point before further differentiation, or to preserve samples for future experiments.
This protocol is for cryopreserving established hepatic organoids in dome cultures grown using the HepatiCult™ Organoid Kit (Human).
Materials
- Healthy hepatic organoid cultures expanded in HepatiCult™ Organoid Growth Medium (Human)
- CryoStor® CS10 (Catalog #07930)
- Advanced DMEM/F-12 (Thermo Fisher 12634028)
- 25% bovine serum albumin (BSA) solution in water
- Sterile 2 mL cryogenic vials
- Sterile 15 mL conical tubes (Catalog #38009)
- Sterile 6-well tissue culture-treated plate (Catalog #38015)
- Sterile 200 µL and 1000 µL pipette tips
- Styrofoam box with ice
- Biohazard safety cabinet
- Controlled-rate cell freezing container (e.g. Corning® CoolCell® (Catalog #200-0642), Mr. Frosty™ etc.)
- Incubator at 5% CO2 and 37°C
Preparation
- Prepare 50 mL of serum-supplemented Advanced DMEM/F-12 (AdvDMEM + BSA):
- 2 mL of 25% BSA
- 48 mL of Advanced DMEM/F-12
- Mix thoroughly and store on ice. Use cold.
Note: This volume is sufficient to cryopreserve one full 24-well plate. If not used immediately, store AdvDMEM + BSA at 2 - 8°C for up to 4 weeks.
- Place CryoStor® CS10 and labeled 2 mL cryogenic vials on ice in the biosafety cabinet.
Protocol
- Check that the Matrigel® domes containing the organoids being cryopreserved are intact. If the dome is intact, proceed to step 2. If the dome is loose, add cold AdvDMEM + BSA to top-up the total volume in the well to 1mL and let sit for 1 minute, then proceed to step 4.
- Without touching the Matrigel® dome, aspirate and discard the medium in each well being processed.
- Using a 1 mL pipettor, forcefully add 1 mL cold AdvDMEM + BSA to the center of each Matrigel® dome and let sit for 1 minute.
- Set the 1 mL pipettor to 1000 µL and vigorously pipette the volume up and down 45 times, taking care not to generate bubbles. This results in mechanical breakdown of hepatic organoids and Matrigel® into smaller fragments of 30 - 100 µm.
Note: Check the sizes of the fragments generated in the well using a light microscope before proceeding to step 5. If most fragments are larger than 100 µm, triturate until they are 30 - 100 µm.
Note: If organoid density is low, or if cryopreserving fragments from multiple wells with the same sample or treatment, the contents of multiple wells can be pooled into a 15 mL conical tube at this stage.
- Vortex the plate containing the organoid fragments in individual wells or the tube containing the pooled organoid fragments gently at medium speed for 5 seconds. To quantify the organoid fragment density in these volumes, immediately transfer 3 x 10 µL from the individual wells or the tube into an empty 6-well plate, creating three separate droplets.
- Using a light microscope, quantify the number of hepatic organoid fragments in each 10 µL droplet, only counting fragments that are 30 - 100 µm. It is recommended to cryopreserve 1000 - 3000 fragments in each cryogenic vial.
Note: If the fragment density is too high to count, dilute the fragment suspension with AdvDMEM + BSA and repeat the count.
- Transfer the desired number of fragments to new 15 mL conical tubes containing 1 mL Advanced DMEM/F-12. Prepare a separate conical tube for each cryogenic vial that will be frozen.
Example:
- Three 10 µL fragment counts: 35, 40, 42
- Average count per 10 µL: 39
- Volume to transfer 1000 fragments to subsequent passage: 256 µL
- Desired number of cryovials containing 1000 fragments: 4
- Prepare 4 x 15 mL conical tubes, each containing 1 mL Advanced DMEM/F-12. Add 256 µL of fragment suspension to each of the four tubes.
- Centrifuge the tubes at 290 x g for 5 minutes.
- Without disturbing the pellets, carefully aspirate and discard as much of the supernatant as possible, leaving 5 - 10 µL in the tube. The pellet is often not visible.
- Place tubes containing pellets on ice. Working with one tube at a time, add 1 mL CryoStor® CS10 and pipette up and down 5 - 8 times to resuspend. Transfer the entire volume into one labeled and cooled cryogenic vial.
- Working quickly, repeat step 10 for all remaining tubes. Transfer all prepared cryogenic vials into a controlled-rate cell freezing container.
- Place cell freezing container at -80ºC for 24 - 48 hours, then transfer cryogenic vials to liquid nitrogen storage.
Note: It is recommended to thaw at least one cryogenic vial within 1 - 2 weeks of cryopreservation to verify viability and density of cryopreserved hepatic organoid fragments.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration