mTeSR™3D: Clump Passaging Method for Suspension Culture hPSCs
- Document # 27053
- Version 1.0.0
- Feb 2017
Introduction
mTeSR™3D has been developed for expansion of human pluripotent stem cells (hPSCs) as aggregates in suspension culture. A clump passaging method is recommended to ensure consistent growth rates from passage to passage, and to minimize the risk of karyotype abnormalities which can be associated with single cell passaging protocols1,2.
2-Stage Process
In the clump passaging method, the hPSC aggregates are:
- Pre-treated with a non-enzymatic reagent, Gentle Cell Dissociation Reagent (GCDR; Cat# 07174).
- Mechanically dissociated to clumps of ideal size for reseeding the next passage using a 37 µm reversible strainer (Cat #27215/27250).
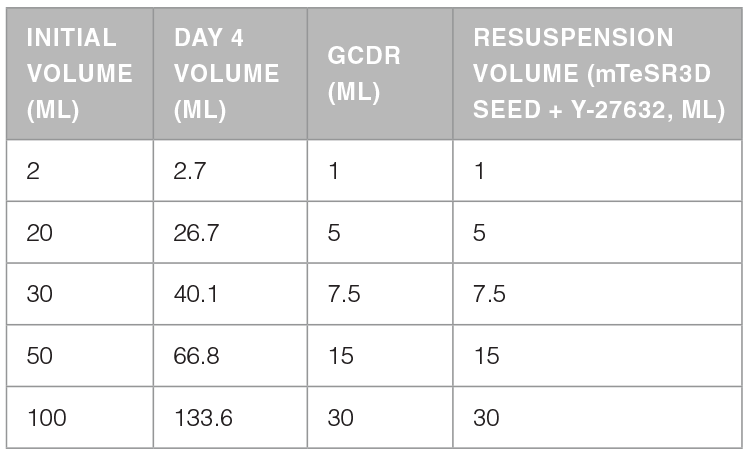
The protocol below is for passaging aggregates from an initial culture volume of 20 mL and a day 4 volume of ~26.7 mL in mTeSR™3D. Volumes can be scaled as required. Please refer to Table 1.
Table 1. Recommended Volumes of GCDR and Resuspension Medium for Clump Passaging
Preparation
- Warm 5 mL of GCDR to 37°C.
- Bring mTeSR™3D Seed Medium to room temperature (15 - 25°C).
- Prepare enough mTeSR™3D Seed Medium + 10 µM Y-27632 ROCK inhibitor to resuspend and seed all conditions. See Table 1 for recommended resuspension volumes.
- Image cultures prior to passaging. Healthy aggregates should be no larger than 350 – 400 µm, and display characteristic morphology including:
- Approximately spherical shape
- Edges that are clear but not perfectly smooth
- A mottled appearance. Shallow craters or pockmarking are normal and are a sign of maintenance of PSC marker expression
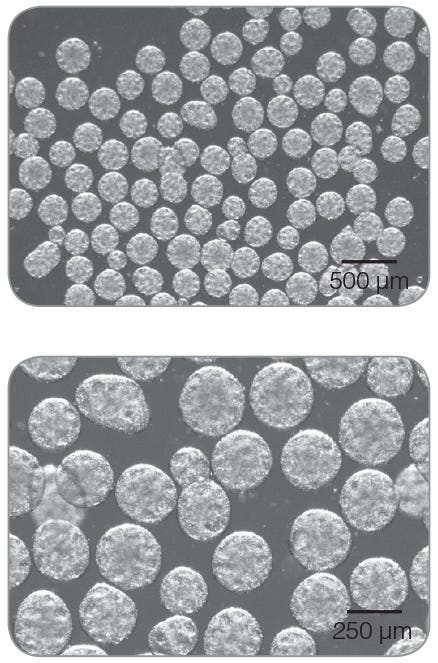
Figure 1. Representative images of hPSC aggregates at the end of a passage in mTeSR™3D. Images taken at 20X (top) and 40X (bottom). Scale bars indicate size.
Stage 1: Pre-Treat hPSC Aggregates with GCDR
- Collect the hPSC aggregates using a serological pipette.
- Filter out non-aggregated single cells by passing through a large 37 µm Reversible Strainer (Cat #27250) into a 50 mL centrifuge tube. Make sure the arrow on the strainer is pointing up (Fig. 2).

Figure 2. Collect the hPSC aggregates using a conical tube and reversible strainer, with the arrow pointing upwards.
- Flip strainer onto a new 50 mL conical tube (Fig. 3).

Figure 3. Flip the reversible strainer onto a new conical tube. The arrow is pointing downwards.
- Rinse the aggregates into the new tube with 5 mL warm GCDR (Fig. 4). If needed, tap the strainer lightly to release all aggregates.
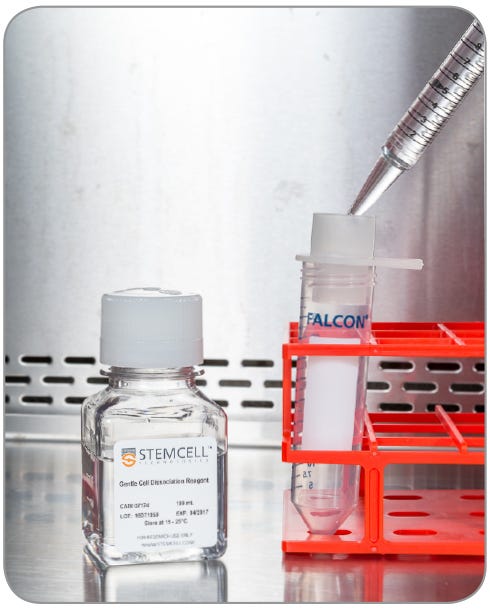
Figure 4. Using warm GCDR, rinse the aggregates into the new conical tube.
- Visually confirm that all the aggregates have been rinsed off the filter.
- Flip the strainer onto a 3rd new 50 mL conical tube and set aside. This strainer will be used again later in the protocol (Stage 2). The arrow will be pointing up again at the end of the flip.
- Incubate the hPSC aggregates and GCDR at 37°C for approx. 6 min, undisturbed. Optimal incubation time may be cell line-dependent.
During step 7, hPSC aggregates are partially dissociated by the GCDR in preparation for mechanical dissociation to small clumps (Stage 2).
- Optional: Centrifuge at 100 x g for 2 min. This may be required to settle the aggregates in the conical tube, if the GCDR volume is > 5 mL.
- Slowly aspirate the GCDR using a serological pipette, leaving ~0.5 mL in the tube. Take care to avoid removing any aggregates.
- Using a serological pipette, add 5 mL mTeSR™3D Seed Medium + 10 µM Y-27632 ROCK inhibitor and gently resuspend the pellet.
Note: If culture has expanded > 5-fold and aggregate density is high, a larger volume of mTeSR™3D Seed Medium + 10 µM Y-27632 ROCK inhibitor may be required. The increase in volume will decrease the shear force and lead to higher viability
Stage 2: Mechanical Dissociation
- Using a 25 mL serological pipette, aspirate the resuspended hPSC aggregates. Ensure there are no bubbles at the end of the pipette tip.
Note: Prevent bubbles by aspirating the aggregate suspension from the tube slowly. Remove bubbles by lightly tapping the pipette to release the bubbles. Try to work quickly once the aggregate suspension is in the pipette to avoid aggregates settling.
- Using the strainer and new tube from Stage 1, carefully place the tip of the pipette containing aggregates directly against the strainer in a vertical orientation (Fig. 5).
- With the slowest setting on the Pipette-Aid, pass the partially dissociated aggregates through the strainer. The flow rate should be ~0.5 - 1 mL per second. This will generate clumps of appropriate size to seed the next passage. Be sure to maintain direct contact between the pipette and strainer throughout (Fig. 5B).
Note: If the strainer appears clogged, stop pipetting and slide the pipette laterally on the strainer while maintaining direct contact, then continue pipetting.

Figure 5. Placement of the serological pipette containing pre-treated aggregates. The pipette should be positioned vertically with the tip in direct contact with the strainer surface.
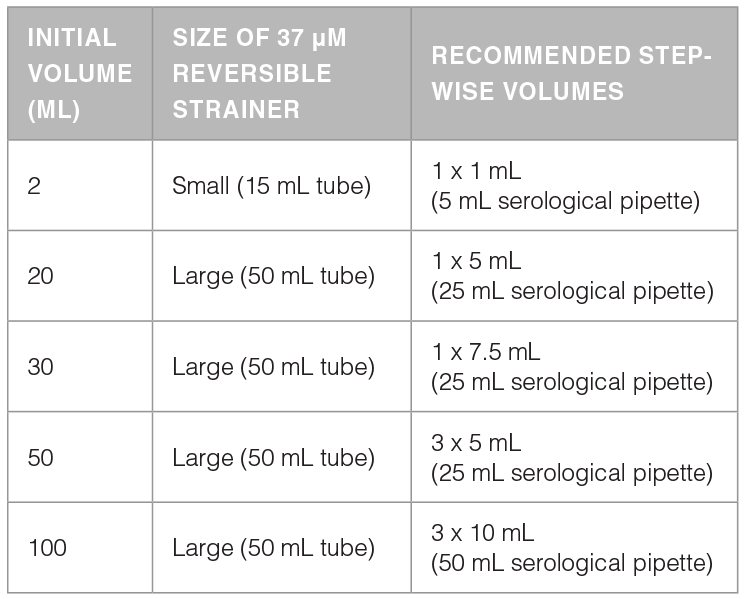
- For larger culture volumes, a stepwise procedure is recommended for mechanical dissociation. Working with small volumes in larger pipettes will control the amount of shear to which the aggregates are exposed. Refer to Table 2.
Table 2. Scaled Protocols for Other Suspension Culture Volumes
- Count viable cells or clumps (see Technical Manual Appendix 1: Counting Clumps). Be sure to gently mix the clump suspension before removing a sample to count.
- Seed a new culture vessel by adding concentrated clump suspension and topping up with additional mTeSR™3D Seed Medium + 10 µM Y-27632 ROCK inhibitor to the desired final volume and cell density.
Note: Clumps may aggregate if left for extended periods of time (> 15 minutes) as a pellet. Gently triturate the entire clump suspension 1 - 2 times using a 25 mL or 50 mL serological pipette at a flow rate of ~1 mL per second immediately before seeding.
For best results:
- Pipette tip is in direct contact with the strainer membrane
- Pipette is vertical
- No air bubbles in the pipette tip
Some hPSC aggregates may remain on the surface of the strainer. Minimize cell loss by adjusting the flow rate and volume of flow-through to suit individual cell lines. To increase yield, rinse the strainer with an additional 1 - 5 mL of mTeSR™3D Seed Medium + 10 µM Y-27632 ROCK inhibitor.
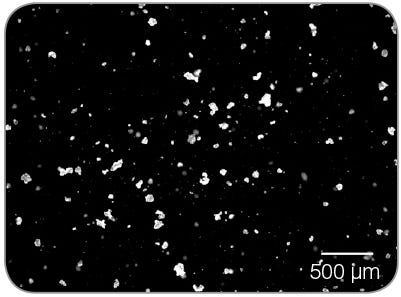
Figure 6. Representative image of newly-generated hPSC aggregate clumps, ~ 50 - 100 µm in size. These hPSC clumps are ready for seeding into the subsequent passage in mTeSR™3D.
Product Info
mTeSR™3D Kit and Required Products
Related Products
hPSC-related Antibodies
References
- Bai Q et al. (2014) Temporal analysis of genome alterations induced by single-cell passaging in human embryonic stem cells. Stem Cells Dev 00(00): 1–10.
- Garitaonandia I et al. (2015) Increased risk of genetic and epigenetic instability in human embryonic stem cells associated with specific culture conditions. PLoS One 10(2): 1–26.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration

