Dr. Ana Camelo describes her work on understanding the role of ILCs in chronic inflammatory diseases
Understanding the Role of ILCs in Chronic Inflammatory Diseases

Dr. Camelo performed her postdoctoral research in Dr. Andrew McKenzie’s lab at the MRC Laboratory of Molecular Biology, where she studied the biology of innate lymphoid cells (ILCs). She then followed her dreams to make a difference in patients’ lives by joining MedImmune, the global biologics research and development arm of AstraZeneca, an innovation-driven biopharmaceutical business that focuses on the discovery, development and commercialization of small molecule and biologic prescription medicines. Ana continued her interest in ILCs in chronic inflammatory diseases at MedImmune.
Topics:
- ILCs in chronic diseases
- Translational ILC research
1. What inspired you to pursue scientific research?
The scientific research “bug” has been with me since I remember. My father, a researcher and engineer, always taught me to question how things worked, and that really developed my curiosity of the world around us. By the age of 6 I was introduced to Newton’s law of gravitation, and at 12 I developed an interest in the human body, especially the blood system. It was then I knew I wanted to be an immunologist.
2. How did you begin your scientific journey?
I started out as an undergraduate reading biochemistry when I was still in Portugal, and moved on to a PhD in Cell biology at the University of Birmingham. I then joined the LMB institute for a postdoc position working on innate lymphoid cells and their role in chronic inflammatory diseases.
3. What is your current role?
I have moved on from academia after my postdoc position into an industry role as I wanted to make a bigger impact in patients’ lives, and have been working at MedImmune for the past 4 years in early biology research, specifically in the development of monoclonal antibodies for the treatment of inflammatory and respiratory diseases.
4. What advice do you have for researchers who are trying to make the transition from academia to industry?
I was very lucky that during my postdoc I did some translation work. That really helped with my transition to industry. I think picking a good lab to do a doctoral degree or postdoctoral research was definitely key in my personal experience. I don’t believe that there is a magic formula that can guarantee a job in industry, but a strong scientific background, a good publication record and an understanding of translational research can definitely make a difference.
Perseverance is always a good quality. It took me several attempts before I was awarded my current position.
5. What do you enjoy the most about your current role?
I really enjoy my current position. Working in a fast-paced environment where we are continuously breaking the boundaries of science to develop therapies that can help patients in need is a real driver for motivation.
6. What has been the biggest thing you learned in your journey?
I think the biggest thing I learned so far in my career is to never underestimate the power of collaboration; sometimes the brightest ideas come from working across unexpected channels.
1. What are the discoveries that have led you to your current work?
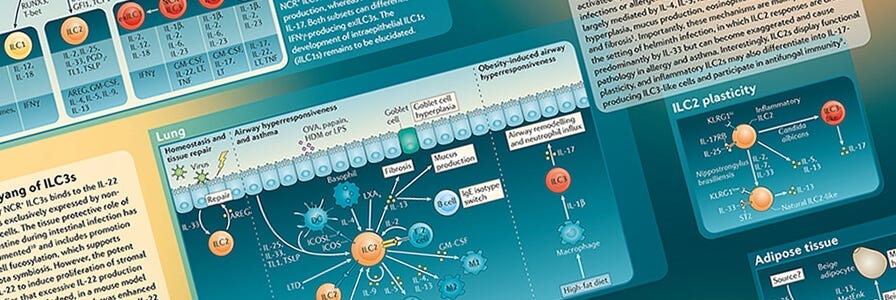
After finishing my PhD in cell biology, I was keen to get back to my favourite subject, immunology and I was very lucky to have been awarded a postdoctoral position at the LMB institute in Cambridge in Andrew McKenzie’s lab. This was when I first started working with innate lymphoid cells (ILCs) and trying to investigate their role in inflammatory bowel disease (IBD) and asthma using pre-clinical models. We were among the first to describe the presence of IL-13-producing ILCs in the gut and how they contributed to disease pathology in a type 2 model of inflammation. We also found that disease could potentially be dampened by treating with anti-IL-25 antibodies1.
2. How do ILC2s and type 2 immunity relate to IBD?
IBD is generally subdivided into two categories: Crohn’s disease and ulcerative colitis. The first is believed to be a more Th1/Th17-skewed disease, whereas ulcerative colitis is believed to have a Th2-skewed phenotype. ILC2s contribute to the ongoing inflammation through their production of the inflammatory cytokine IL-13. IL-13 has been pointed as one of culprit cytokines driving the process of fibrosis, especially in the lungs, however it is not yet known if ILC2s could directly promote fibrosis in IBD. Recent publications have pointed to a role of ILC2s in lung fibrosis2 via their activation through IL-25 and to a role for ILC3s driving fibrosis in a Crohn’s disease mouse model3. More work needs to be done to dissect their exact role and contribution to inflammation in these chronic diseases.
3. Tell us about your current research. What have you found so far?
I moved on from my postdoc to a Research Scientist position at MedImmune where I continued developing my understanding of ILCs in respiratory disease, and how upstream cytokine signaling can affect their phenotype and function. I developed a method for isolation of these cells from human peripheral blood and culture conditions which allowed me to understand their biology more deeply, and how they can respond differently to cytokines such as IL-33, IL-25 and TSLP4.
4. What is the significance of your research?
Understanding the role that ILCs play in chronic diseases is important. As a previously unidentified cell type, immunology has mostly focused on T cells and aimed therapeutics at targeting T cells. However, ILCs can produce much higher amounts of cytokines like IL-5 and IL-13 than T cells, which could potentially have a higher impact on the recruitment of other cell types such as neutrophils, eosinophils and macrophages. These effector cells can in turn cause an extensive amount of damage if their numbers and activation status are increased in the mucosal tissues (such as lung, gut, etc). Although ILCs represent a very small population in the blood, their potential to induce a lot of damage by perpetuating the inflammatory cascade is significant.
5. What are the complexities that you are facing in this field?
I think the main challenge is trying to understand the role ILCs play in the mucosal system in humans. This is complicated for obvious reasons, as it is hard to source human tissue to run these experiments. Pre-clinical models, although an invaluable resource, are not always representative of what is happening in humans, especially in a disease setting that can be very complex.
The fact that they are such a rare population of cells makes it really important to have a robust method of isolation and culture, as even different media (without the addition of cytokines) can induce a skewed phenotype in these cells, an observation which I mentioned in more detail in our ILC publication4. This could confound our interpretation of in vitro mechanisms and how they correlate to their real behaviour in the tissue, particularly in a disease setting.
Understanding how ILCs behave in the tissue, where they are localised and which cells they can come directly in contact with is currently a challenge. We are making big steps in this direction though so I think a lot more understanding of ILC biology in situ is going to start emerging in the near future.
6. Where do you think the field is heading and what’s next for your research?
As science makes progress in treating allergic diseases and chronic inflammation, we must work to further our understanding of the mechanisms of action of potential new therapies. I believe we will find that ILCs can be targeted by these therapies in the future and that doing so can help ameliorate the chronic inflammatory symptoms in patients.
7. Since ILCs are a newly discovered cell type, existing drugs previously thought to work through a certain mechanism of action may actually work through their effects on ILCs. Are you aware of any examples?
This is an interesting point. Although we do extremely thorough research during the research and development process—working to understand the detailed mechanism of action of therapies—evolving scientific advances do make it difficult to test all possibilities. We always know more today than we did yesterday.
In saying that, I do believe that therapies that have been developed to target particular cytokines, such as IL-33, IL-25 and TSLP, could have an impact on ILC2 cell number, activation status and/or phenotype; and indirectly on other cell types that cross-talk with ILC2s, such as T cells, B cells and mast cells, and others. In the future, researchers pursuing therapeutics that target these pathways may need to start taking this cell type into account when trying to understand the mode of action of their drugs.
1. Why do you need to isolate cells for your research?
In the ILC field, having a high purity population to work with is extremely important. As a rare cell type, if there are other cell contaminants in the culture it has the potential to confound the end results of the experiment. A good pre-enrichment of ILCs is key to an effective and successful (high purity) cell sort.
2. Which cell isolation products have you used?
Throughout my career, I have used many STEMCELL isolation kits: for isolation of T cells, NK cells, B cells, monocytes, DCs, etc, depending on which project I work on.
EasySep™ Cell Separation
Isolate highly purified immune cell subsets in as little as 8 minutes with a simple pour.
View more Immunology Profiles or explore selected publications showcasing how cells isolated with EasySep™ are used.
References
- Camelo A et al. (2012) Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. J Gastroenterol 47(11): 1198-211.
- Hams E et al. (2013) IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci USA 111(1): 367-372.
- Lo BC et al. (2016) The orphan nuclear receptor RORα and group 3 innate lymphoid cells drive fibrosis in a mouse model of Crohn’s disease. Sci Immunol 1(3): eaaf8864.
- Camelo A et al. (2017) IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv 1: 577-89.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration