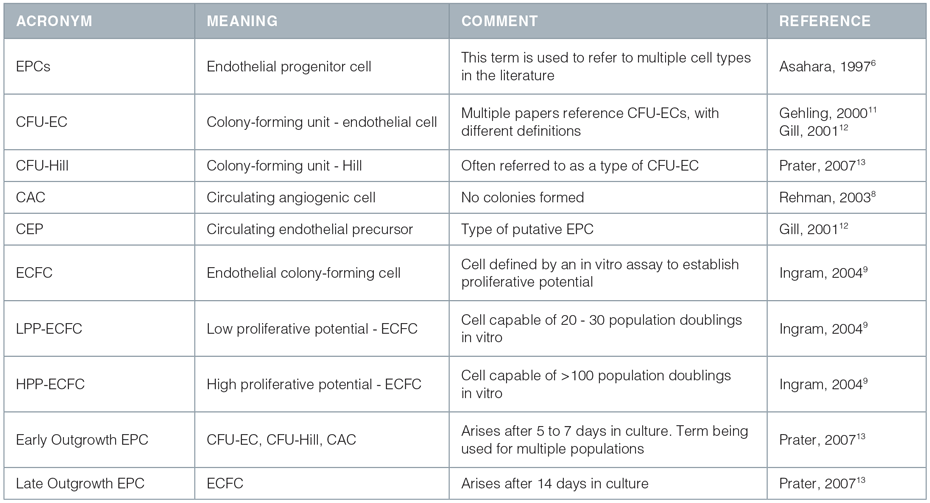
Endothelial Cells, Angiogenesis, and Vasculogenesis
Blood vessel development is a regulated process involving the proliferation, migration, and remodeling of endothelial cells (ECs) from adjacent pre-existing blood vessels (angiogenesis) or following differentiation of endothelial progenitor cells (EPCs) from mesodermal precursors (vasculogenesis)
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
- Document # 29012
- Version 4.0.0
- Jan 2013
Blood Vessel Development and Endothelial Progenitor Cells
Blood vessel development is a regulated process involving the proliferation, migration, and remodeling of endothelial cells (ECs) from adjacent pre-existing blood vessels (angiogenesis) or following differentiation of endothelial progenitor cells (EPCs) from mesodermal precursors (vasculogenesis).1,2 EPCs were originally thought to be present only during embryonic development. However evidence accumulated in the past several years suggests that they can persist in the bone marrow and/or in circulation into adult life. This has generated interest in the use of EPCs for neovascularization of ischemic or injured tissue and for the clinical assessment of risk factors for various diseases.3-5
Terminology
The field of adult vasculogenesis research is young and evolving. The term 'EPC' was originally used in 1997 based on work performed by Asahara et al.6 that proposed the existence of a cell type with endothelial progenitor function. Asahara's group isolated a heterogeneous population of circulating cells which reportedly differentiated into cells expressing markers of ECs in vitro, had proliferative activity, and contributed to new vessel formation in animal models of ischemia. Those cells with vasculogenic potential in vivo were believed to give rise to endothelial colonies in vitro and were termed EPCs. More recently, many distinct populations of cells that appear to correlate with or influence postnatal vasculogenesis have been identified, and these distinct cells have all been referred to as EPCs.1,3,7-10 Subsequently, the term 'EPC' has been used to describe different cell populations by different authors. A summary of the use of the term 'EPC' is given in Table 1. It should be noted that some of these cells may be more properly referred to an "angiogenic cells", i.e. cells that support or augment angiogenesis and/or vasculogenesis, without actually differentiating into cells that form part of the vascular network.
Phenotypic Characterization Of EPCs
The heterogeneity in cell types referred to as EPCs has made the identification of definitive EPC markers difficult. Studies to purify and characterize EPCs from bone marrow (BM) or peripheral blood (PB) have been hampered by the absence of markers to phenotypically distinguish these cells from mature vascular wall-derived ECs and from subsets of hematopoietic cells. Many of the characteristics associated with EPCs, including LDL uptake, lectin binding, and CD31/105/144 expression, are also found on monocytes, making the distinction between putative EPCs and monocytes especially difficult.11 Numerous populations of cells appear to contribute to the formation of blood vessels, either by direct incorporation into vascular networks or indirectly, possibly in a character in manner, including CD34,6 CD133 and VEGFR2 positive cells,12 subsets of monocytic cells,13 and cell populations with broad developmental plasticity such as multipotent adult progenitor cells (MAPCs).14
The specific markers defining a true EPC, differentiating intermediates, and mature ECs are not known. Neither has the phenotype of circulating angiogenic cells, those which contribute to vasculogenesis and angiogenesis indirectly, been fully clarified. Some papers suggest that the loss of CD133 expression represents a good marker to distinguish between an endothelial progenitor and a mature endothelial cell.1,15 However, other work16 suggests that cells triply positive for CD133, CD34 and VEGFR2 do not form endothelial cell colonies, at least under the culture conditions used, but do form hematopoietic colonies under hematopoietic cell colony conditions. Nor did these triply positive cells form capillary-like structures on Matrigel™. Interestingly, CD34+ CD45- cells did form endothelial cell colonies and capillary-like structures in the respective assays, suggesting that true EPC may express CD34 and lack expression of CD45.16
Subtypes of Putative EPCs, Angiogenic and Vasculogenic Cells
EPC and Angiogenic Cell Assays
As an alternative to characterizing EPCs and angiogenic cells by cell surface antigens or markers, some investigators have defined these cells based on their different growth properties in vitro.
A cell culture assay has been developed by Hill et al.3 to assess the correlation between the frequency of a specific population of circulating cells, clinical factors, and vascular function.
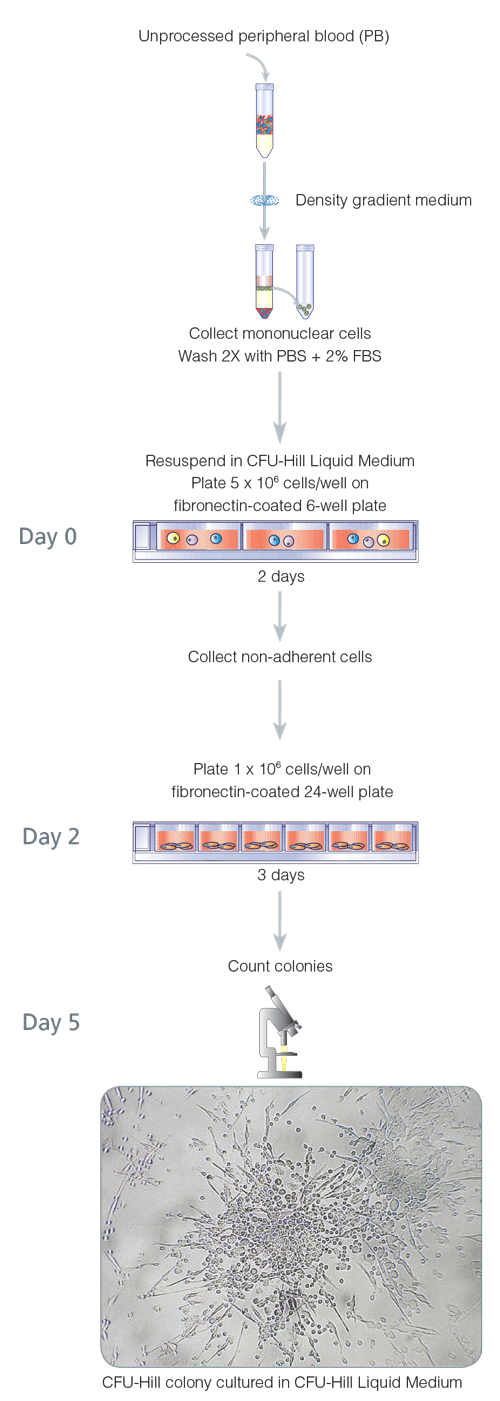
In this widely used assay, peripheral blood mononuclear cells are plated in fibronectin-coated dishes for two days, to remove some adherent cell populations. After two days, the nonadherent cells (which contain the cells of interest) are harvested and replated on fibronectin-coated dishes. Colonies are evaluated and quantified 3 days later. In this assay, positive colonies are defined as a central core of "round" cells, with more elongated"sprouting" cells at the periphery. Immunohistochemical staining has confirmed that cells generated in this assay express the endothelial markers von Willebrand factor, VEGFR2 and CD31.3 Hill et al., using the assay, found that in healthy individuals, the number of colonies negatively correlated with the Framingham cardiovascular risk score and positively correlated with vascular function, as measured by flow-mediated brachial artery reactivity.3 STEMCELL Technologies has standardized this 5 day assay and refers to it as the 5 Day CFU-Hill Colony Assay (Figure 1), in an attempt to distinguish this assay from other CFU-EC and EPC assays. Unique colonies that are formed in the 5 Day CFU-Hill Colony Assay are referred to as colony-forming unit-Hill colonies or CFU-Hill colonies. The CFU-Hill Liquid Medium Kit has been developed specifically to support the growth and quantification of CFU-Hill colonies. A growing list of associations between CFU-Hill frequency and various disease states, as described in the 'EPC, Angiogenic cells, and Disease States' section highlights the utility of this assay.
Ingram et al., (working with Yoder), have developed an alternative assay that quantifies colonies described as 'endothelial colony-forming cells' (ECFCs) and determines the proliferative potential of the cells that forms the colony.9 In this assay, peripheral blood mononuclear cells are plated on collagen I-coated dishes in medium containing endothelial growth factors. In contrast to the cell (or cells) that form the 5 Day CFU-Hill colony, the cell (or cells) that produces ECFC is rapidly adherent as all nonadherent cells are removed and discarded with frequent medium changes. Colonies are evaluated after 14 to 21 days in culture. Using the ECFC assay, adult PB was found to contain cells with the ability to proliferate for 20 - 30 population doublings, which were termed low proliferative potential endothelial colony-forming cells (LPPECFC).
Cord blood, however, contained cells with the ability to form colonies after 100 population doublings, which were termed high proliferative potential endothelial colony-forming cells (HPPECFC). The colonies themselves contained cells expressing an array of EC surface proteins. The development of such assays,which evaluate the self-renewal and proliferative capacity of putative EPCs, will be very helpful in determining the phenotype of the EPC and its differentiation pathway.
Recent work has partially clarified the relationship between ECFCs and the cells giving rise to CFU-Hill colonies. Yoder et al.17 showed that ECFC-derived cells express many EC antigens, including CD31, CD105, CD144, CD146, VWF, KDR, and UEA-1. Some, but not all, cells generated in the 5 Day CFU-Hill Colony Assay were shown to express the same markers, and cells from both assays incorporated AcLDL. Cells in the CFU-Hill colonies, but not cells derived from ECFCs, were found to express the hematopoietic marker CD45 and the monocyte markers CD14 and CD115.
The progeny of CFU-Hill colonies were able to ingest and kill microbes, a characteristic of macrophages but not of ECs. In addition, cells from CFU-Hill colonies could not be replated to form secondary CFU-Hill colonies, while cells from ECFC colonies could form secondary ECFC colonies. When plated in methylcellulose containing hematopoietic growth factors, cells from CFU-Hill colonies formed myeloid colonies. When cells from both assays (CFU-Hill and ECFC) were plated in collagen/fibronectin gels and implanted in NOD/SCID mice, cells from ECFC colonies but not cells from CFU-Hill colonies gave rise to neovessels containing human endothelial cells.
Together, this suggests that CFU-Hill progeny are hematopoietic derived monocytes and macrophages, and not EPCs or ECs. This study clearly distinguishes ECFC from CFU-Hill, and it has been suggested the term EPC is more appropriate for cells giving rise to ECFC colonies than to cells giving rise to CFU-Hill colonies. CFU-Hill colonies, however, remain potent biomarkers of vascular health and correlate with many disease states, as described in the section 'EPC, Angiogenic Cells, and Disease States'.
Figure 1. 5-Day CFU-Hill Colony Assay
Mechanisms to Promote Vascular Homeostasis
The mechanisms by which each different cell population contributes to vasculogenesis remains unclear, but likely include direct contributions via proliferation and integration as endothelial cells (EPC), and indirectly via secretion of angiogenic growth factors, recruitment of EPCs, or modification of the extracellular matrix (angiogenic cells). For example, transplantation studies performed on immuno-deficient mice demonstrated that "early outgrowth EPCs" synergized with "late outgrowth EPCs" to restore blood flow to the limbs of immuno-deficient mice with hind limb ischemia, indicating that early outgrowth cells do contribute to neoangiogenesis.18 It is likely that many cell populations work together during neovascularization. Thus while numerous sources of putative EPCs provide measurable proangiogenic function after transplantation, they may not directly produce endothelial cells or be integrated into the endothelium proper.19 Recent work by Purhonen et al.20 using several in vivo models suggests that BM-derived cells are always perivascular in location and never form part of the vasculature.20 While CFU-Hill colonies may not include cells that can integrate into neovasculature, correlations between CFU-Hill frequency, cardiovascular risk factors, and cardiovascular function clearly show that the cells that produce these colonies play some function in vascular homeostasis. The CFU-Hill assay has, to date, been predominantly used to study vascular perturbations in disease states (i.e. as a biomarker of vascular homeostasis). In contrast, the ECFC assay may be more useful for the production of therapeutic cell populations.
EPC, Angiogenic Cells, and Disease States
There has been no systematic study of the number of EPCs present in healthy individuals. However, several studies have described the influence of pathological conditions, drugs, and growth factors on "EPC" number in vivo (not exactly the same cells in each study). For example, the number of circulating EPCs and their migratory activity was reportedly decreased in patients with risk factors for coronary artery disease4 or negatively correlated with the Framingham cardiovascular risk score.3 EPCs from patients with diabetes mellitus type 2 have been characterized by a decreased proliferative capacity, reduced adhesiveness, and reduced ability to form capillary tubes in vitro.21 The mechanism(s) responsible for these findings is unknown but may be attributed to a decreased mobilization of EPCs, an increased consumption of EPCs at the injury site, and/or a reduced half-life of EPCs. In contrast, limb ischemia22 and acute myocardial infarction23 were associated with a rapid increase of EPCs in the circulation. Treatment with different hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) inhibitors (statins)24,25 and a number of growth factors including EPO,26 VEGF,27 and GM-CSF22 have also been reported to increase the number of EPCs in vivo. Preliminary evidence suggests that they may act by mobilizing EPCs from the bone marrow and/or may improve the survival of EPCs by activation of the PI3 kinase/Akt pathway.2 The ECFC assay9 has been used in studies on patients with coronary stenosis. Guven et al.28 found an increase in the concentration of ECFCs in relation to the severity of cardiovascular disease. A number of recent reports have used the 5 Day CFU-Hill Colony Assay and/or CFU-Hill Medium (Catalog #05900) in investigations into colony frequency and coronary artery disease,29 peripheral arterial disease,30 chronic obstructive pulmonary disease,31 asthma32 and stroke.33
Conclusions
Regulation and assessment of neovasculogenesis in adults is vital to the understanding and treatment of many diseases. Numerous cell populations play a role in vascular homeostasis, and although significant progress has been made in this exciting area of research, it is evident from the literature that there are inconsistencies in the terminology and definitions used in this field. Clear and widely accepted definitions of specific terms and standardized procedures to isolate, phenotypically characterize and culture EPCs and other angiogenic cell populations are necessary to advance the field of endothelial/angiogenic research further. Such consistency is a prerequisite for the use of EPCs and angiogenic cell populations in the development of therapies and for quantification of such populations as a diagnostic tool in clinical studies.
Reference
- Rafii S, et al., Nat Med 9: 702-712, 2003
- Masuda H, et al., Cardiovasc Res 58: 390-398, 2003
- Hill JM, et al., N Engl J Med 348: 593-600, 2003
- Vasa M, et al., Circ Res 89: e1-e7, 2001
- Mancuso P, et al., Blood 97: 3658-3661, 200
- Asahara T, et al., Science 275: 964-966, 1997
- Werner N, et al., N Engl J Med 353: 999-1007, 2005
- Rehman J, et al., Circulation 107: 1164-1169, 2003
- Ingram DA, et al., Blood 104: 2752-2760, 2004
- Kalka C, et al., Proc Natl Acad Sci USA 97: 3422-3427, 2000
- Rohde E, et al., Stem Cells 24: 357-367, 2006
- Peichev M, et al., Blood 95: 952-958, 2000
- Schmeisser A, et al., Cardiovasc Res 49: 671-680, 2001
- Reyes M, et al., J Clin Invest 109: 337-346, 2002
- Schmeisser A, et al., J Hematother Stem Cell Res 11: 69-79, 2002
- Case J. et al., Exp Hematol 35:1109-1118, 2007
- Yoder MC, et al., Blood 109: 1801-1809, 2007
- Yoon CH, et al., Circulation 112: 1618-1627, 2005
- Zentilin L, et al., Blood 107: 3546-3554, 2006
- Purhonen S, et al., PNAS 105: 6620-6625, 2008.
- Tepper OM, et al., Circulation 106: 2781-2786, 2002
- Takahashi T, et al., Nat Med 5: 434-438, 1999
- Shintani S, et al., Circulation 103: 2776-2779, 2001
- Vasa M, et al., Circulation 103: 2885-2890, 2001
- Llevadot J, et al., J Clin Invest 108: 399-405, 2001
- Heeschen C, et al., Blood 102: 1340-1346, 2003
- Kalka C, et al., Circ Res 86: 1198-1202, 2000
- Guven H, et al., J Am Coll Cardiol 48: 1579-1587, 2006
- Thum T, et al., Am Coll Cardiol 46: 1693-1701, 2005
- Shaffer RG, et al., Cytometry B Clin Cytom 70: 56-62, 2006
- Palange P, et al., Eur Respir J 27: 529-541, 2006
- Asosingh K, et al., J Immunol 178: 6482-6494, 2007
- Sobrino T, et al., Stroke 38: 2759-2764, 2007


