Using Stem Cell-Based Models in Drug Discovery

In this interview, Dr. Bas Trietsch shares his experiences as Chief Technology Officer at MIMETAS, a biotech company that offers organ-on-a-chip solutions. He talks about the importance of human-based 3D model systems, their impact on drug discovery, and the future outlook of this technology.
Watch the Full Interview >
Published August 20, 2024
The following transcribed interview has been lightly edited for clarity and brevity.
Bas’ Inspiration: Impacting Patient Lives
Tell us about MIMETAS and your current role there.
MIMETAS is a biotech company in the Netherlands that offers organ-on-a-chip solutions to the pharmaceutical industry. We work with pharma to help them kickstart and improve their drug discovery processes by offering best-in-class human disease models. We're always happy to work with our partners, to give them the right models that reflect a physiologically relevant environment, thus helping researchers achieve their goals and answer their questions with higher fidelity and throughput. As one of the co-founders of MIMETAS, I've always been pushing our capabilities and technology, which include microfluidic and biological products, automation and screening capabilities, data sciences, and everything else we need to achieve our joint goals with our partners faster.
What inspired you early in life to pursue a career in science and led you to the biotech field?
I've always been really interested in figuring out how things work. The most complex thing to figure out is the human body and all the intricate processes that are ongoing there, leading to either healthy or pathophysiological states. So science has always been close to my heart.
During my PhD, which was very much focused on lab-on-a-chip, I got in touch with the right people and with the right applications and realized that doing chemistry is only so much fun. Having the capability of impacting patient lives, helping cure diseases, and helping bring therapies to patients who have no other outlook to becoming healthy again, is something very easy to be motivated about and easy to get up every morning for.
The Drug Discovery Landscape: The Need for Physiologically Relevant Models
What challenges are your partners facing that are leading them to explore more advanced in vitro tissue models and add those to their research toolkits?
We've tried for the last half a century or so to use classical in vitro and in vivo research techniques to gain a better understanding of diseases. But to a large extent, that has proven an inefficient or sometimes dead-end endeavor. Our conviction at MIMETAS is that this is all down to a fundamental problem—if you use the wrong model to test your new drugs, if you use the wrong systems to do your early research or your late-stage safety and efficacy assessments, you'll get the wrong answers.
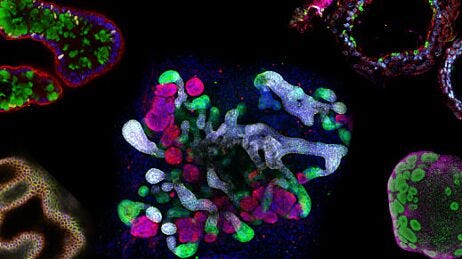
It's a process that has proven problematic because it's not like a funnel, it's much more like a maze. If at the start, you take the wrong avenues down that maze, it's difficult to course-correct. We’ve found treatments for diseases in mice and cured cancer in a vacuum multiple times over, but to actually address pathophysiological mechanisms in the full human system requires human-relevant models. This is where people come to us—they need better models. In our mind, better models mean 3D systems, always human-based, with multiple different cell types together in an architecture that recapitulates the physiological situation to the best of our abilities. That often involves human stem cells and organoids as a base material, but taking these organoids and putting them in the right context: vascularizing them, perfusing them with compounds, and including different cell types (Figure 1).
We try to take all of the pieces of this complex interplay that make up a full pathobiological process and place them in an assay that you can modulate and study without sacrificing throughput—the other pillar of what we always want to have in our models. We want to have best-in-class biology with the right interaction between cell types but at reasonable throughput. That's why our OrganoPlate® system is a 384-well plate. It allows you to easily screen tens of thousands of compounds and do high-fidelity, human-relevant research at scale, enabling people to change their perspective on their research and unlock new questions and new areas, and gain new insights.

Figure 1. Advanced Human 3D Models Can Be Derived from Human Pluripotent Stem Cells (PSCs) and Tissue Biopsies
Human-relevant 3D models are the key to understanding the pathophysiological mechanisms of organ tissues. PSCs and tissue samples undergo expansion and differentiation pathways to produce organoids that can be pre-vascularized and co-cultured with endothelial and other supporting cells. When placed in a microfluidic-based platform, the in vivo functionality of the tissue can be recapitulated to study disease mechanisms and identify potential drug targets.
What steps have you seen firms take to start using advanced in vitro models as smoothly as possible?
It's one of the core design principles that we've always tried to stay true to; if we come up with a complex, cumbersome, and non-scalable solution to one of the questions we have, we simply say, no, let's think: how can we get best-in-class biology without sacrificing scalability?
We need to ensure compatibility with all the infrastructure that people have already invested in. Take something that's for an end user, and tailor it to biologists who can just use these tools as they would any other research tool. We've taken it a step further to cater to different types of partners. So we do a lot of work for customers where we take your question, put it into our innovation engine, take the biological resources that we already have at our fingertips, and incorporate them into models so that we can return with an answer.
The latest kit we have is called OrganoReady® plates, which are plates that already have live biology on them. If you want a plate full of colon organoid tubules, we can send it to you precultured. You just take off the blister and do your experiment. That makes this starting point for end users really easy. We want to help users incorporate this type of technology into their drug discovery pipeline quickly and easily in whatever mode of operation fits their needs the best.
Can you comment on the value of systems that incorporate human stem cell-based biological elements versus other models such as immortalized cell lines?
I think there is a place for different models, right? There is a place in the world for immortalized cell lines, they have a huge legacy and an immense body of literature available, which adds value to any observations you do with your compound in a small system like Caco-2 cells. They’re very well-defined, very well-known, and can be used to answer very specific questions. You have great context, but they can also be an inherently limiting type of cell to work with. They have their uses but lack the interplay and complexities between the different aspects of human biology that you may not be aware of, which can be relevant to the models that you're looking at.
We believe that what's going to give you new insights is the unforeseen interplay between different proteins in one cell type, but also between different cell types interacting with each other in their response to pharmacological interventions. To get as close as possible to an in vivo situation, you want to have multiple cell types that can interact in the same way they do in the body because then you actually get the full picture. That's where advanced in vitro biology can make a big difference, versus more simple molecule-to-molecule assays, immortalized cell lines that lack certain functionalities, or models that have the wrong expression levels of different enzymes and proteins that can send you on a wild goose chase.
I think the same can be said for using stem cell-based human biology and maintaining the right phenotype so you can see what is happening, instead of zooming into one small little aspect and being blind to any unforeseen interaction or any emergent properties that come from a more complex interplay between cells and their environments.
To get as close as possible to an in vivo situation, you want to have multiple cell types that can interact in the same way they do in the body because then you actually get the full picture. That's where advanced in vitro biology can make a big difference…
Dr. Bas Trietsch
Has legislation, such as the FDA Modernization Act 2.0 and the EU’s commitment to modernize science, had an impact that you can see through your work with pharma companies at MIMETAS?
There are interesting dynamics surrounding these legislative changes. Some people were looking at this on the surface and thought it was going to be obligatory or they were suddenly going to be allowed to do these types of things. But in effect, advanced 3D biological models were already allowed. The early adopters, those looking for the best, and most modern ways of approaching drug discovery, were already doing this before the FDA Modernization Act. But it woke up the other parts of pharma that were biding their time more. I think those are the people that got shaken up a little bit and thought, “Okay, this is not a fad. This is something that we should look into and get on board in our processes because this is the future.” And that's what we've seen primarily. It's been helpful in basically making best-in-class biology the talk of most biotech executives out there.
It also got even the more conservative researchers out there thinking about it. We've seen that it's a good conversation starter, and it makes everybody realize a lot of the possibilities that maybe were not forbidden before, but are now much more in front of them and perceived as more mainstream.
Stem Cell-Based Model Systems: Our Solution
What are some of the more significant challenges that the companies you're working with are facing?
In making the change to more advanced models, there's confusion in the market where there are a lot of different offerings that have variable levels of “technology readiness”. I think what we've been focused on, and what has been helped by companies like STEMCELL, is taking best-in-class biology and making it robust and reliable, not experimental. These are complex, advanced models using best-in-class biology. If you use validated cell sources and media that are well-defined and compatible with the biology you're trying to achieve, you can place that in systems that are made for screening to generate data that is reproducible, reliable, and scalable. If you don't approach it with those types of platforms, you're going to be in trouble because it's going to become an experiment for the sake of using an advanced model.
What we are on board for is to solve difficult questions, and that's not about having the most fancy model. It's about being able to capture the complexity so that you gain new understanding and insights, and can break through some of the barriers that conventional models cannot.
What are some applications of advanced in vitro models and where along the discovery process can they be used?
Target discovery: This is something that we typically approach from a phenotypic angle. We like to focus on comprehensive models, modulate them using a range of compounds, and try to match them with certain phenotypical or functional changes that we observe in our biology. We often do that with high-throughput screening, where we can also use different AI-based tools to do largely unsupervised morphometric and phenotypic analysis. We can then start to match the phenotype and map them together with the a priori knowledge of how these compounds work and reconstruct the mechanism of action. The next step of validating these targets is usually easier because then you know what you're looking for. If you already have a target, you can use CRISPR, you can use siRNAs. What we then try and do is have a handful of orthogonal assays that confirm which targets are crucial in getting the right pathophysiological intervention.
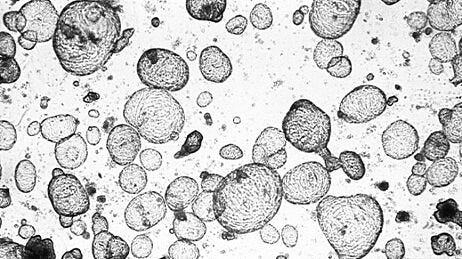
Mechanistic Toxicity Studies: We have done quite some work on compound rescue—finding compounds that had early or late stage failure, trying to figure out the mechanism of toxicity, and seeing if we can fix those issues—but also taking compounds and understanding if they could be useful in a different context, if they could be useful for a certain subsection of patients (Figure 2). Going back to organoid models where we have different donors and patient samples, we can look at this very high-fidelity representation of specific patients and see if this failure just needs more patient stratification to select the right patient group to help. Those are the types of research that we have been doing. It can be quite interesting because you start close to the finish line, right? So they can be quite exciting projects.

Figure 2. Drug-Repurposing Screens on Patient-Derived Organoids Can Open New Avenues for Patient Care
A promising strategy in developing novel therapies is drug repurposing: the identification of new applications for existing compounds. Drug repurposing can be done using patient-derived organoids (PDOs) which play a pivotal role in validating the efficacy of the given compound in different disease contexts. This allows for the development of tailored therapies for select patient groups, widening the options for treatment in patient care.
Immuno-Oncology: Our platform is unique in that we have full vascularization and perfused multicellular tissues with no membranes or physical barriers between different aspects of the tissue. We have engineering control over where we place cells to start with. After they form into a tissue, cells are free to move, interact, and change positions as they wish. That means that we have a strong focus on transport, but also on migration processes. This means that immuno-oncology is an exciting field for us now. We can have fully vascularized tissue, tumors, the stromal compartments, and perfuse them with CAR T or just normal immune cells, and see how we can modulate the homing of those cells to those tumors in a real, complete way. A similar approach can be taken to look at metastases, using circulating tumor cells or rare circulating tumor cells, and see if we can modulate how they extravasate or home.
Inflammatory & Fibrotic Diseases: Another aspect where cell-cell interaction and these gradients are important is in inflammatory and fibrotic disease areas and applications. This is usually early on in the drug discovery pipeline, but being able to fully visualize cell-cell interactions at scale with no artificial separation or interactions, is something that opens up avenues not possible without using this type of technology. Doing that with the best starting materials, usually stem cell-derived, often organoids, is a toolbox that usually scientists are pretty excited to get their hands on. It enables them to answer questions that they've stared at for a long time but lacked the tools to unpack.
What are some of the most impactful gains that firms are seeing when they are choosing to take this step and explore incorporating these more advanced in vitro models into their systems?
There are two sides that we usually see. One is a model for a process that was completely untouchable before. For example, if you want to look at metabolic dysfunction-associated steatohepatitis (MASH), you accept that this is a complex interplay between all the different cell types. You want to have a vascularised liver model with hepatocytes, stromal cells, and resident and circulating immune cells and have all of them interact with each other in 3D. There's just no other way to look at that complete picture, right? So that's just one example of assays that are just not possible any other way. Using this type of technology, for the first time you're able to approach a certain type of biological process.
On the other side, it's also about taking an assay that's already there but making it more translatable, sensitive, and reliable. If we stay in the liver field from the MASH example, we need to have proper long-term maintained metabolic activity, but also the regenerative properties of these tissues—regenerative properties not only being relevant for the liver but also for things like the colon. If we're doing IBD-type research, we look at not only the interplay between fibroblasts, epithelial cells, and immune cells, but also how these get damaged, recover after an insult, and maybe maintain sensitivity to the two new inflammatory cues. That type of sensitivity and fidelity is something that maybe before was only achievable at scale and at a level of reproducibility using irrelevant cell types, but that just means that you're not in the right ballpark, not looking at the things you want to study. So I think the most impactful gains are, not only having reliable data at scale, but the data also being meaningful. In the end, that's what will get you to the right answers for your research questions a bit faster.
Wallchart: Dynamic Modeling in Organoids
Learn more about organoid applications for studying human health and disease.
Get Your Free Copy >Advice and Future Outlook
What do you see as the next evolution of organ-on-a-chip system use in general or the types of systems that are being generated at MIMETAS?
At MIMETAS, we’re always expanding our portfolio and trying to push the limit of the biological fidelity of our models. We always want to have more patient relevance and comprehensive, complete models to use at scale.
In general, what we see as a relatively recent push is to deploy the amazing advances in AI to unlock and leverage highly information-dense phenotypic models fully. Moving away from what can be a pretty limiting classical image analysis, through 3D versions of cell paintings, all the way to a fully comprehensive analysis. You can take all of the data that we gather from different screens, and use that to train AI models. From there, you can take a new compound, do an assay on it, and interpret that in the context of all of the data that we've already fed into our algorithms. This can allow you to understand what you see happening in a very detailed and sensitive manner in something that doesn't have to be a huge screen.
What is the importance of a collaborative effort between technology developers, end users, and regulatory bodies in the advancement of new approach methods (NAMs)? How do you see the importance of those types of conversations happening in the next little while?
The collaboration with pharma and with regulators is absolutely crucial. In the end, that's where all roads lead, right? That's where all the research needs to funnel towards, to have a drug reach a patient. Being fully aligned is something that is really, really important. We are in the luxurious position of being a company that has the engineers making the technology behind these models. Two-thirds of my colleagues are the scientists who are doing this research and they interact daily with pharma to try and address their problems. That's where we can really, in a very efficient and fast turnaround manner, deploy all of the tools that we have available to us and also develop new tools to enable progress there as fast as possible. It would be good if that type of interaction happens more across the board because there's a lot out there; I'm not jealous of pharma having to look at these hundreds of different offerings for which it's pretty difficult to distinguish between a new idea of a bright PhD student and an established technology that has been proven and is directly deployable at scale.
If I had to pick the biggest challenge for the field of organ-on-a-chip at the moment, it's confused customers who have so many options to pick from that they don't know where to start. We've been in this field for a long while and we've built our brand on being a reliable partner that delivers good data for really advanced questions. I think the challenge for the whole field is going to be, how do we take all of these bright minds that are all doing new different approaches, and bundle our strengths to be able to offer pharma the right solution for their ask at that moment, without having to do an evaluation of 200 different technologies before they even start.
Related Resources
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration