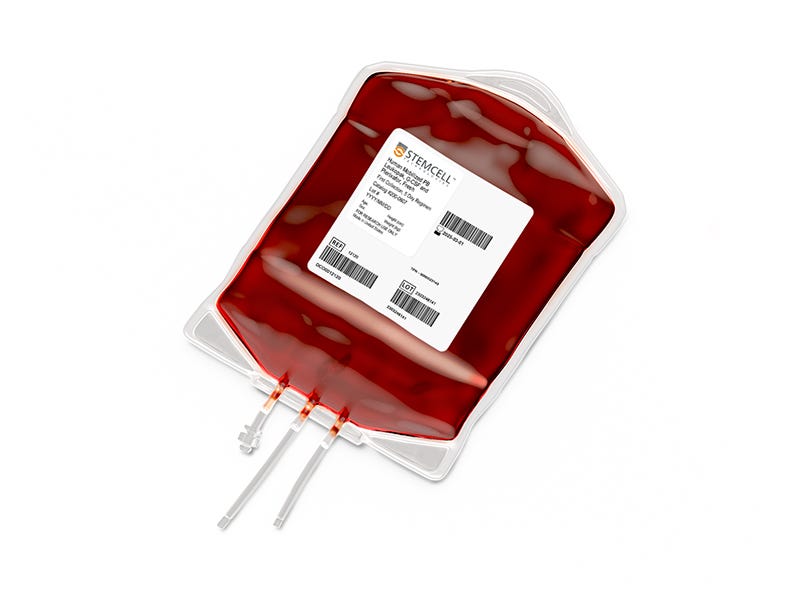
Obtain large numbers of single-donor CD34+ hematopoietic stem and progenitor cells (HSPCs) from peripheral blood leukopaks mobilized with granulocyte colony-stimulating factor (G-CSF), plerixafor, or G-CSF and plerixafor (Combo). Using fresh mobilized leukopaks as a rich source of circulating CD34+ cells from a single donor minimizes donor-to-donor single-cell variability, ensuring consistency across your experiments and allowing scalability of your stem cell studies.
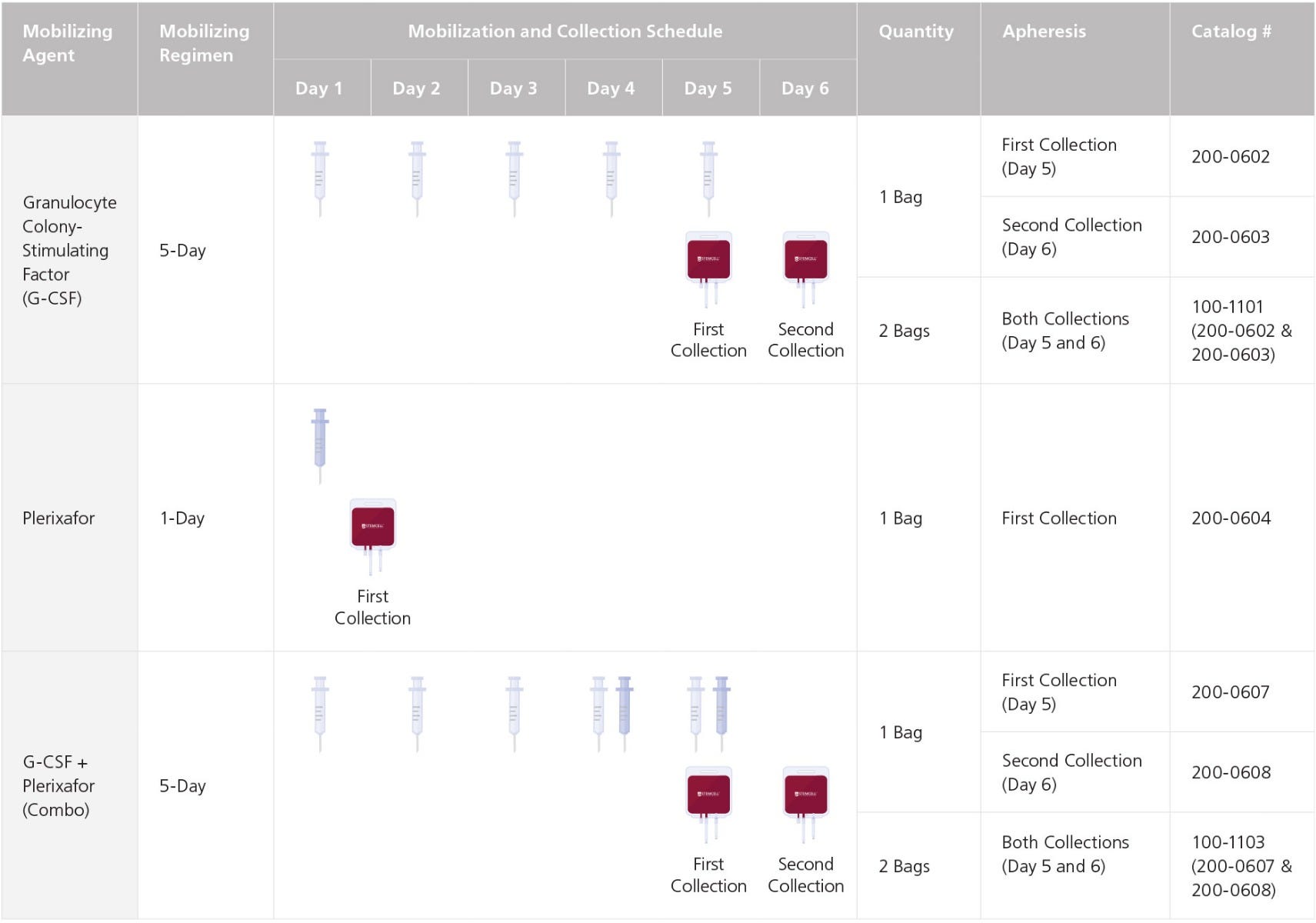
Donors are mobilized with one of the following:
• G-CSF (NEUPOGEN®) or filgrastim: a maximum of 10 µg/kg/day of G-CSF for 5 days prior to collection
• Plerixafor (Mozobil®): a maximum of 0.24 mg/kg plerixafor for 1 day prior to collection
• G-CSF and plerixafor: a maximum of 10 µg/kg/day of G-CSF for 5 days prior to collection and a maximum of 0.24 mg/kg of plerixafor for 1 - 2 days prior to collection
Each leukopak is a highly concentrated, low-volume apheresis collection from a single, normal donor, using Institutional Review Board (IRB)-approved consent forms and protocols. A full-sized leukopak is produced from ~2 - 3x blood volumes using the Spectra Optia® Apheresis System, with acid-citrate-dextrose solution A (ACDA) as the anticoagulant. Donors are screened for HIV-1, HIV-2, hepatitis B, hepatitis C, human T-lymphotropic virus types I and II (HTLV-I/-II), syphilis, and West Nile Virus (WNV). The product will be shipped with a Certificate of Analysis (CoA) containing the reported total nucleated cells, viability, CD34+ cell frequency, standard donor information, HLA results, and full flow cytometry panel showing viable cells, CD34+ cells, monocytes, B cells, NK cells, and CD4+ and CD8+ T cell populations.
Certain products are only available in select territories. Please contact your sales representative or Product & Scientific Support at techsupport@stemcell.com for further information.
Browse our
Frequently Asked Questions (FAQs) on Primary Cells.
NEUPOGEN® and Mozobil® are registered trademarks of Amgen Inc. and Genzyme Corporation respectively.