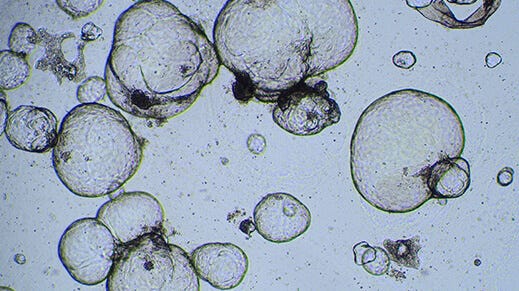
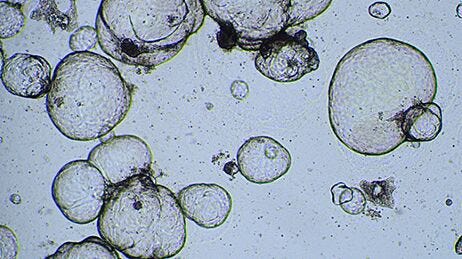
How to Perform Cytotoxicity Screening Using Hepatic Organoids Cultured in HepatiCult™ (Human)
Assessment of hepatotoxicity is a critical element of preclinical drug development in order to reduce the risk of failure in later stages of the process. Due to their ability to faithfully model human tissue complexity and functionality, organoids are increasingly being incorporated into preclinical screening programs to supplement or replace traditionally used systems, including immortalized and primary cell lines and animal models. As such, organoids provide a convenient, scalable, and powerful tool for predicting the cytotoxic effects of potential therapeutics in a human-specific context.
The below protocol describes a hepatotoxicity screening assay using human liver organoids grown with the HepatiCult™ Organoid Kit (Human).
Materials
- HepatiCult™ Organoid Growth Medium (Human) (Catalog #100-0385)
- HepatiCult™ Organoid Differentiation Medium (Human) (Catalog #100-0383)
- Healthy hepatic organoid cultures (12 - 24 confluent domes in a 24-well plate are required to seed one full 96-well plate)
- 25% bovine serum albumin (BSA) solution in water
- Corning® Matrigel® (Corning® Catalog #356231, ≥ 8 mg/mL protein)
- Antibiotics (e.g. gentamicin)
- Advanced DMEM/F-12 (Thermo Fisher Catalog #12634010)
- DMEM/F-12 with 15 mM HEPES (Catalog #36254)
Preparation
- Warm Corning® 96-well plate in the 37°C incubator overnight.
- Thaw HepatiCult™ Organoid Growth Supplement at room temperature (15 - 25°C) or overnight at 2 - 8°C. Prepare 100 mL of complete HepatiCult™ Organoid Growth Medium (OGM) using sterile technique, as follows:
- Combine 95 mL HepatiCult™ Organoid Basal Medium and 5 mL HepatiCult™ Organoid Growth Supplement
- Add antibiotics (e.g. 50 μg/mL gentamicin)
- Mix thoroughly. Warm complete HepatiCult™ OGM to room temperature before use.
Note: Once thawed, use HepatiCult™ Organoid Growth Supplement immediately or aliquot and store at -20°C. After thawing aliquots, use immediately. Do not re-freeze. - Thaw HepatiCult™ Organoid Differentiation Supplement at room temperature (15 - 25°C) or overnight at 2 - 8°C. Prepare 100 mL of complete HepatiCult™ Organoid Differentiation Medium (ODM) using sterile technique, as follows:
- Combine 95 mL HepatiCult™ Organoid Basal Medium and 5 mL HepatiCult™ Organoid Differentiation Supplement
- Add antibiotics (e.g. 50 µg/mL gentamicin)
- Mix thoroughly. Warm complete HepatiCult™ ODM to room temperature before use.
Note: Once thawed, use HepatiCult™ Organoid Differentiation Supplement immediately or aliquot and store at -20°C. After thawing aliquots, use immediately. Do not re-freeze. - Prepare 50 mL BSA-supplemented Advanced DMEM/F-12 (AdvDMEM + BSA), as follows:
- Combine 2 mL 25% BSA solution in water (1% final concentration) and 48 mL Advanced DMEM/F-12
- Mix thoroughly. Keep on ice during use.
- Thaw ~700 µL of Matrigel® on ice (preferably overnight at 2 - 8°C) to seed one full 96-well plate. This includes an extra 20% volume to account for loss during pipetting.
- (Optional) Hepatic organoid fragments may adhere to the surfaces of conical tubes and serological pipettes. To minimize adherence, pre-wet 4 x 50 mL and 2 x 15 mL conical tubes with Anti-Adherence Rinsing Solution and Advanced DMEM/F-12, as follows:
- Transfer 5 mL of Anti-Adherence Rinsing Solution to the 15 mL conical tubes and 10 mL of Rinsing Solution to the 50 mL conical tubes.
- Tighten the lids and swirl tubes to coat. Transfer the Anti-Adherence Rinsing Solution to the remaining tubes, swirling to coat each one.
- Aspirate Anti-Adherence Rinsing Solution from tubes.
- Repeat the previous steps with 5 mL Advanced DMEM/F-12 for the 15 mL conical tubes and 10 mL of Advanced DMEM/F-12 for the 50 mL conical tubes, then aspirate residual Advanced DMEM/F-12.
- Cap all coated tubes and store on ice until required.
Organoid Dissociation
- Check that the Matrigel® domes to be passaged are intact (i.e. the whole dome remains attached to the plate and no loose Matrigel® pieces or organoids are observed in the well). If the domes are intact, proceed to step 2. If the domes are loose, add cold AdvDMEM + BSA to top up the total volume in the well to 1 mL, then proceed to step 4.
- Without touching the dome, aspirate and discard the medium in each well to be passaged.
- Add 1 mL of cold AdvDMEM + BSA to each well.
- Using a 1 mL pipettor, vigorously pipette the total volume in the well up and down 45 times, taking care not to generate bubbles.
Note: This results in mechanical dissociation of the organoids and Matrigel® into smaller fragments of 30 - 100 µm. Check fragment sizes using a light microscope; if most fragments are larger than 100 µm, triturate further until they are ≤ 100 µm.
- Collect all dissociated organoid fragments in a 50 mL conical tube and keep on ice.
- Wash each well with 1 mL cold AdvDMEM + BSA and add this wash volume to the dissociated fragments in the conical tube.
(Optional) Fragment Size Filtration
Note: Filtration of dissociated fragments may help reduce variability in experimental setup.
- Place a 100 µm reversible strainer on a new 50 mL conical tube, with the arrowhead on the strainer pointing downward. Pre-wet the surface of the strainer with 2 mL Anti-Adherence Rinsing Solution followed by 2 mL AdvDMEM + BSA. Discard the flow-through and transfer the strainer to a new pre-wetted 50 mL tube.
- Using a 10 mL serological pipette, transfer 10 - 20 mL of the fragment suspension through the 100 µm strainer. Fragments smaller than 100 µm will pass through, while fragments larger than 100 µm will be retained on the membrane.
- Wash fragments larger than 100 µm off of the strainer membrane as described below.
- Reverse the 100 µm strainer onto a new pre-wetted 50 mL conical tube.
- Add 2 - 4 mL of AdvDMEM + BSA directly onto the membrane to wash off all fragments.
- Replace the strainer on the 50 mL conical tube containing the flow-through and continue with step 4.
- Transfer an additional 10 - 20 mL of fragment suspension through the strainer. Repeat steps 2 and 3 until the entire volume of fragment suspension has been passed through the strainer. Place the flow-through, containing fragments that are smaller than 100 µm, on ice.
- (Optional) To maximize the yield of fragments available to seed domes, fragments that are larger than 100 µm can be triturated further as follows:
- Aliquot 1 mL of the suspension containing fragments larger than 100 µm (collected in step 3) into each well of a new 24-well plate.
- Using a 1 mL pipettor, vigorously pipette the total volume in the well up and down 45 times, taking care not to generate bubbles.
- Pass the triturated fragment suspension through another pre-wetted 100 µm strainer and place the flow-through on ice. Pool this flow-through with the flow-through collected in steps 2 - 4.
- Place a 37 µm reversible cell strainer on a 50 mL conical tube. Pre-wet the surface of the strainer membrane with 2 mL of Anti-Adherence Rinsing Solution followed by 2 mL of AdvDMEM + BSA. Discard the flow-through and transfer the strainer to a new 50 mL tube (this tube does not need to be pre-wetted).
- Using a 10 mL serological pipette, transfer 10 mL of the fragment suspension collected through the 37 µm strainer. Single cells and fragments smaller than 37 μm will pass through the filter, and fragments 37 - 100 µm will be retained on the membrane. Discard the flow-through.
- Reverse the 37 µm strainer onto a new pre-wetted 50 mL conical tube. Add 2 - 4 mL of AdvDMEM + BSA directly onto the membrane to wash off all fragments between 37 - 100 µm, placing the pipette tip directly onto the membrane to dislodge the fragments. Discard the strainer.
- Repeat steps 6 - 8 until the entire volume of fragment suspension collected in steps 2 - 4 has been passed through a 37 µm strainer, and all fragments between 37 - 100 µm have been collected and pooled into the 50 mL tube used in step 8. Place this tube on ice.
Seeding
- Determine the number of organoid fragments using droplets in a 6-well plate, as follows:
- Using a light microscope, count the number of organoid fragments in 3 x 10 µL droplets. Count all fragments.
- Calculate the concentration of fragments per mL.
Example:- Three 10 µL fragment counts: 35, 40, 42
- Average count per 10 µL: 39
- Average fragments per mL: 3,900
- Transfer the volume required to plate the desired number of wells, adding 20% extra volume, to a 15 mL conical tube.
Note: Seeding density should be optimized per donor. We recommend seeding 150 - 400 fragments per 6 µL dome per well of the 96-well plate.
- Centrifuge tube(s) at 290 x g for 5 minutes. Aspirate as much of the supernatant as possible without disturbing the pellet.
- Resuspend the fragments in 6 µL Matrigel® per dome to be seeded, plus an extra 20% to account for pipetting loss. Mix gently with a 1 mL pipettor, taking care not to generate bubbles. Place tubes on ice.
- Remove the pre-warmed 96-well plate from the incubator and place it in a biosafety cabinet.
- Pipette 6 µL of the fragment-Matrigel® suspension into the center of each well to form a dome. After plating every 6 - 12 domes, return the fragment-Matrigel® suspension to ice for 30 seconds. Mix gently before proceeding with plating an additional 6 - 12 domes.
- Place the lid on the culture plate. Carefully place the plate in an incubator at 37°C and 5% CO2 for 10 minutes to allow the domes to solidify.
- Remove the plate from the incubator and place it in the biosafety cabinet.
- Without disturbing the domes, carefully add 200 µL of room temperature complete HepatiCult™ OGM against the side of wells containing domes. Do not pipette directly onto the domes.
- Add sterile phosphate-buffered saline (PBS) or water to any unused wells. Place the lid on the culture plate.
- Incubate the plate at 37°C and 5% CO2 for 4 - 5 days.
- At 4 -5 days after seeding, cytotoxicity screening using organoids in HepatiCult™ OGM can be initiated, or organoids can be further differentiated in HepatiCult™ Organoid Differentiation Medium (ODM) by performing full-medium changes using HepatiCult™ ODM every 2 - 3 days for 7 days before initiating cytotoxicity screening as described below.
Cytotoxicity Screening
- Prepare stock solutions of the compounds to be tested in the appropriate solvent (e.g. DMSO), using vendor-reported solubility values as a reference.
- Serially dilute these stock solutions 1 in 4 in the appropriate solvent (e.g. DMSO) to generate a 10-point dilution series. Use these dilutions as 100x stocks.
Note: Aliquots of compound stocks can be stored at -20℃. Do not re-freeze more than 3 times.
- Prepare experimental plate layout. Use 4 wells of the 96-well plate per compound concentration, such that 2 compounds can be screened per plate. Use 8 wells each for the solvent/vehicle control and “no treatment” control. Refer to Figure 1 for a sample plate layout.
1 2 3 4 5 6 7 8 9 10 11 12 Compound 1 A No treatment Solvent Control Dose 1 Dose 2 Dose 3 Dose 4 Dose 5 Dose 6 Dose 7 Dose 8 Dose 9 Dose 10 B No treatment Solvent Control Dose 1 Dose 2 Dose 3 Dose 4 Dose 5 Dose 6 Dose 7 Dose 8 Dose 9 Dose 10 C No treatment Solvent Control Dose 1 Dose 2 Dose 3 Dose 4 Dose 5 Dose 6 Dose 7 Dose 8 Dose 9 Dose 10 D No treatment Solvent Control Dose 1 Dose 2 Dose 3 Dose 4 Dose 5 Dose 6 Dose 7 Dose 8 Dose 9 Dose 10 Compound 2 E Dose 1 Dose 2 Dose 3 Dose 4 Dose 5 Dose 6 Dose 7 Dose 8 Dose 9 Dose 10 No treatment Solvent Control F Dose 1 Dose 2 Dose 3 Dose 4 Dose 5 Dose 6 Dose 7 Dose 8 Dose 9 Dose 10 No treatment Solvent Control G Dose 1 Dose 2 Dose 3 Dose 4 Dose 5 Dose 6 Dose 7 Dose 8 Dose 9 Dose 10 No treatment Solvent Control H Dose 1 Dose 2 Dose 3 Dose 4 Dose 5 Dose 6 Dose 7 Dose 8 Dose 9 Dose 10 No treatment Solvent Control Figure 1. Sample Cytotoxicity Screening Plate Layout
- Perform a 1 in 100 dilution of the 100x stock compounds to prepare complete organoid media to treat organoids as follows:
- For compound treatments, dilute the stocks prepared in step 2 with complete OGM or ODM, preparing sufficient volumes for all wells that will be treated.
- For the solvent control wells, dilute the appropriate solvent (e.g. DMSO) in complete OGM or ODM, preparing sufficient volumes for all wells that will be treated.
- Transfer the 96-well plate(s) of organoids to be treated to the biosafety cabinet.
- Without touching the domes, aspirate and discard the medium in each well manually.
- Add 100 µL of the appropriate medium supplemented with compounds or solvents (prepared in step 4) to the wells, and add complete OGM or ODM to the “no treatment” wells.
- Incubate at 37°C and 5% CO2 for 24 hours.
- Repeat steps 4 - 8 two more times, for a total of three treatments. Analyze cell viability 24 hours after the last compound treatment, for a total treatment duration of 72 hours.
- Thaw CellTiter-Glo® 3D reagent overnight at 2 - 8°C. Warm to room temperature before use, inverting gently to mix.
- Warm DMEM/F-12 to room temperature before use.
- Equilibrate the 96-well plate of treated organoids to room temperature for approximately 20 minutes.
- In a conical tube, combine mixed CellTiter-Glo® 3D reagent and DMEM/F-12 at a 5:3 ratio (i.e. 50 µL CellTiter-Glo® 3D + 30 µL DMEM/F-12 per well). Mix gently by inverting or pipetting up and down slowly.
- Without touching the domes, aspirate and discard the medium in each well.
- Add 80 µL of CellTiter-Glo® 3D + DMEM/F-12 to each well.
- Pipette up and down several times to dissociate the organoids and Matrigel®.
- Incubate the plate on a heated benchtop orbital shaker at 25°C and 120 rpm for 30 minutes.
- Transfer 55 µL of the contents of each well to a white-walled plate (e.g. VWR Catalog #82050-726).
- Record luminescence.
- Plot non-linear regression curves using the log of the compound concentration and measured relative luminescence unit (RLU) values (as a percentage of the solvent control) to generate dose-response curves and determine the IC50.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration