Q&A: How to Optimize Your T Cell Therapy Workflow—Without the Use of Serum or Feeder Cells
Meet the Speakers

Dr. Dominika Nackiewicz
Dr. Dominika Nackiewicz, Product Manager for Immunology and Immune Cell Culture Applications at STEMCELL Technologies, helps to convert new ideas into innovative, reliable products. Dr. Nackiewicz received her PhD in Experimental Medicine from The University of British Columbia and received additional immunology training at the University of Virginia and Yale University.

Jessie Yu
Dr. Jessie Yu is a Senior Scientist at STEMCELL Technologies, where she has spent 14+ years developing new products in immune cell research. Her expertise ranges from immune cell isolation to human T cell activation and expansion. She received her MD in 2001 from Shandong University and MSc in Immunology in 2006 from the University of British Columbia. With a background in medicine and immunology research, Dr. Yu firmly believes in adoptive cell therapy as a breakthrough tool to treat many diseases. She is committed to introducing innovative products and applications to the field.

Tim Le Fevre
Tim Le Fevre is a Senior Scientist working as part of the Immunology – Culture & Applications team within the Research & Development department at STEMCELL Technologies. He has worked at STEMCELL for more than 10 years developing cell culture products to support the growth and differentiation of various immune cells. Recently, Tim worked on developing STEMdiff™ and StemSpan™ immune cell kits that enable researchers to differentiate human pluripotent stem cells or cord blood-isolated hematopoietic stem and progenitor cells into monocytes, T cells, or NK cells.
The development of safe and efficacious T cell therapies requires a wide range of reliable tools and technologies. To avoid costly delays and ensure a smoother transition from bench to bedside, scientists should use standardized reagents for their T cell cultures. In this talk, Dr. Dominika Nackiewicz will introduce how STEMCELL Technologies can help optimize your T cell cultures—with robust, high-performance products and expert support. You will learn how to generate high yields of functional T cells for cell therapy manufacturing using the new GMP ImmunoCult™ medium and activators. She will also discuss efficient gene editing strategies using the new CellPore™ Transfection System and solutions for T cell generation from pluripotent stem cells or hematopoietic progenitors.
Q&A Report
Are the ImmunoCult™ activators and medium compatible with other commercially available reagents?
Jessie Yu
Thank you for the question. We designed the activator and medium to be optimized to work together, so we would of course recommend testing them together first. However, we do understand that sometimes you have to run reagents with other reagents. Internally, we have done some studies in this regard, but please reach out to our Sales team for additional information for a case-by-case discussion.
What is the benefit of using a serum-free and feeder-free medium?
Tim Le Fevre
Serum itself is a very complex biological mixture, likely containing a lot of different unknown and advantageous factors, and this means you’re going to have to do a lot of lot-to-lot variability screening to find a serum that will work well for your application. This variability also makes it much more difficult to have reproducible findings in your work. Feeders, at the same time, add a bit more complexity to your culture. They need to be maintained separately and taken care of, and this will add further regulatory hurdles if you’re trying to use a system that has feeders involved in it. So, avoiding all these things is the main benefit of having a serum- and feeder-free medium, and we certainly recommend that.
What is the difference between CD3/CD28 and CD3/CD28/CD2 T cell activator in terms of cell activation and expansion?
Jessie Yu
That’s a great question! With the time constraints, we could only introduce the results we generated with CD3/CD28/CD2 T cell activator. However, we have tested both of them internally. They both support activation and expansion of T cells. Usually, the CD3/CD28 activator is gentler than the CD3/CD28/CD2 activator. With CD3/CD28, you may see a different activation dynamic and also T cell growth pattern. Also, you may see a different CD4/CD8 ratio after expansion, as those are our large datasets. Please contact our Sales team for more information. We would love to share that with you.
What is the expected ratio of CD4+ and CD8+ cell populations in the final product?
Jessie Yu
For the primary T cell expansion question, as Dominika mentioned, there are many parameters that can influence your final product, especially its CD4 and CD8 ratio, such as the choice of activator and medium, the culture vessels, and your selected donors and patients. In general, we find that CD3/CD28/CD2 activators tend to maintain the CD4/CD8 ratio in the starting population, whereas CD3/CD28 activators tend to selectively favor CD8 cell population expansion. So again, just reach out to us and we can provide you with more details.
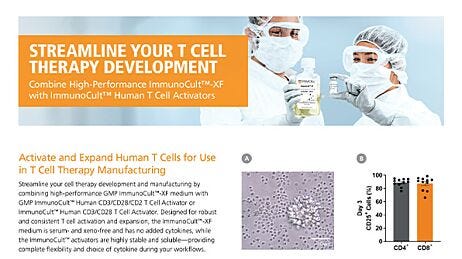
Streamline Your T Cell Therapy Development
Confidently expand T cells without the need for serum or serum derivatives, in xeno-free and phenol red-free ImmunoCult™-XF medium. Achieve robust T cell activation and expansion by combining high-performance GMP ImmunoCult™-XF with GMP ImmunoCult™ human T Cell activators.