Cell Preparation Matters
How do you ensure reliable and timely crossmatch results?
The complement-dependent cytoxicity (CDC) crossmatch assay and the flow cytometric crossmatch (FCXM) assay are some of several screening tests performed to facilitate solid organ transplants. The crossmatch assays specifically assess whether the recipient has antibodies against the donor’s human leukocyte antigen (HLA) type. Detection of these donor-specific anti-HLA antibodies (DSA) is used as part of the risk assessment for transplant rejection.
The crossmatch assays may be the final tests performed before the transplant—providing reliable results in a timely manner is critical. Obtaining crossmatch assay results faster means a shorter turnaround time that may in turn translate to reduced graft ischemia time.1 Isolating cells from whole blood, splenic tissue, or lymph nodes is one of the upstream steps for the crossmatch assay and an important factor in delivering reliable and timely results without compromising sensitivity.
The Importance of Sample Preparation
How cell purity may affect your crossmatch results
Detection of DSA using the crossmatch assay requires recipient serum and lymphocytes or T and B cells from the organ donor. One common technique to isolate cells from donor blood samples for the crossmatch assay is density gradient centrifugation. However, obtaining cells using this method will result in a final cell population that contains a mix of lymphocytes and monocytes. In addition, as the blood sample ages, granulocytes, red blood cells (RBCs), and platelets change density and granulocytes degranulate. These changes may result in granulocyte and RBC contamination when processing older blood samples (> 24 hours post collection) using the density gradient centrifugation method. Dr. Robert Liwski, Medical Director, HLA Laboratory at the Queen Elizabeth II Health Sciences Centre in Halifax, noted that a mixed cell population containing lymphocytes, monocytes, and granulocytes may affect the sensitivity of crossmatch results.2 He assessed the impact of donor lymphocyte purity on FCXM assay results and found that:
- Improved lymphocyte purity resulted in better, faster, and easier events acquisition, gating, and overall analysis.
- Using a cell preparation of pure lymphocytes improved donor-specific antibody (DSA) detection and reduced variability of the FCXM results.
- Using cell preparations with low lymphocyte purity could result in missing weak DSA detection when using the FCXM assay.
- Lymphocyte purity has an overall significant impact on the FCXM results.
Using Highly Purified Lymphocytes Improved DSA Detection
To measure the impact of lymphocyte purity, the FCXM assay was performed with either whole leukocytes (WL) containing lymphocytes, monocytes and neutrophils, or with highly purified lymphocytes (Ly). Use of Ly improved DSA detection while reducing variability of the FCXM results (see figure below).
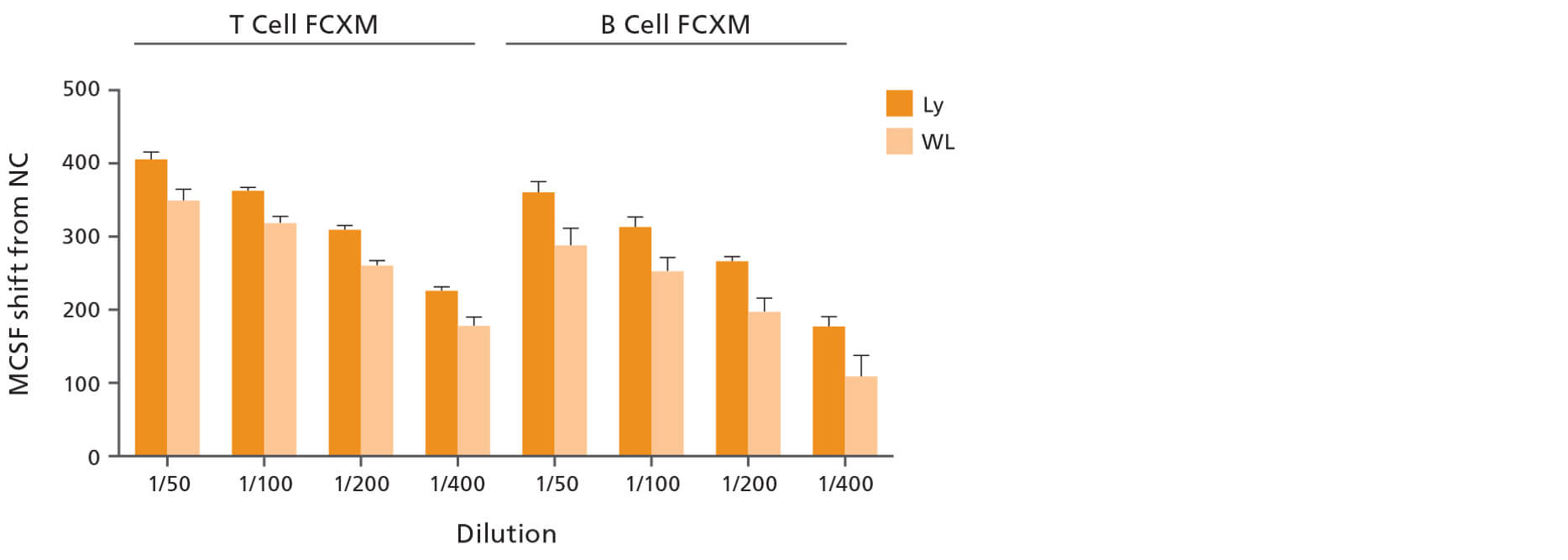
Figure 1. Use of Highly Enriched Lymphocytes (Ly) Isolated with EasySep™ Direct Improves DSA Detection of FCXM Results Compared to Whole Leukocyte (WL) Cell Preparations
Lymphocytes (Ly), neutrophils (Nu), and monocytes (Mo) were isolated from volunteer donors (n=5) using EasySep™ Direct Catalog #19655, #19666, and #19669, respectively. Whole leukocyte (WL) preparations were obtained by adding Ly, Nu, and Mo cells in equal propotions. WL (low lymphocyte purity) and Ly (high lymphocyte purity) preparations were treated with pronase and then used to perform the FCXM assay against negative control sera or several dilutions of positive control sera. The median channel fluorescence shifts (MCFS) were generated by using the negative control sera samples as a baseline. The MCF shifts between WL and Ly were then compared. Each column with error bars represents the mean ± SEM (n = 5 donors). Figure taken from Development and Validation of a Rapid Optimized Flow Cytometry Crossmatch (FCXM) Assay.
Deliver Crossmatch Results Faster
Reduce the length of the cell isolation step
The crossmatch assay is a critical test performed before an organ transplant and faster crossmatch results can allow the clinical team to start organizing transplant logistics sooner. A limiting factor for delivering faster crossmatch results is the time required to isolate cells from the donor’s samples, a necessary step upstream of the crossmatch assay itself.
The method and the time involved in the cell isolation step will depend on the tissue and specific cell populations that are isolated. The FCXM assay typically uses total lymphocyte populations (T and B cells) isolated from whole blood and spleen. The CDC crossmatch assay can be performed with T cells and B cells that are obtained from blood, spleen, or lymph nodes. Choosing an efficient technology to reduce the length of the cell isolation step can dramatically reduce the overall time it takes to perform the crossmatch assay and obtain final results.
The Halifaster Protocol
To reduce turnaround time and standardize the FCMX assay, Dr. Robert Liwski, Medical Director of the HLA Laboratory at the Queen Elizabeth II Health Sciences Centre in Halifax, developed the Halifaster Protocol. This rapid optimized FCXM assay protocol reduces the overall time to complete the assay by over 50%—to less than 2 hours—without compromising quality or sensitivity. This reduced protocol length is in part due to a faster cell isolation step that uses immunomagnetic technology to obtain lymphocytes directly from blood samples.
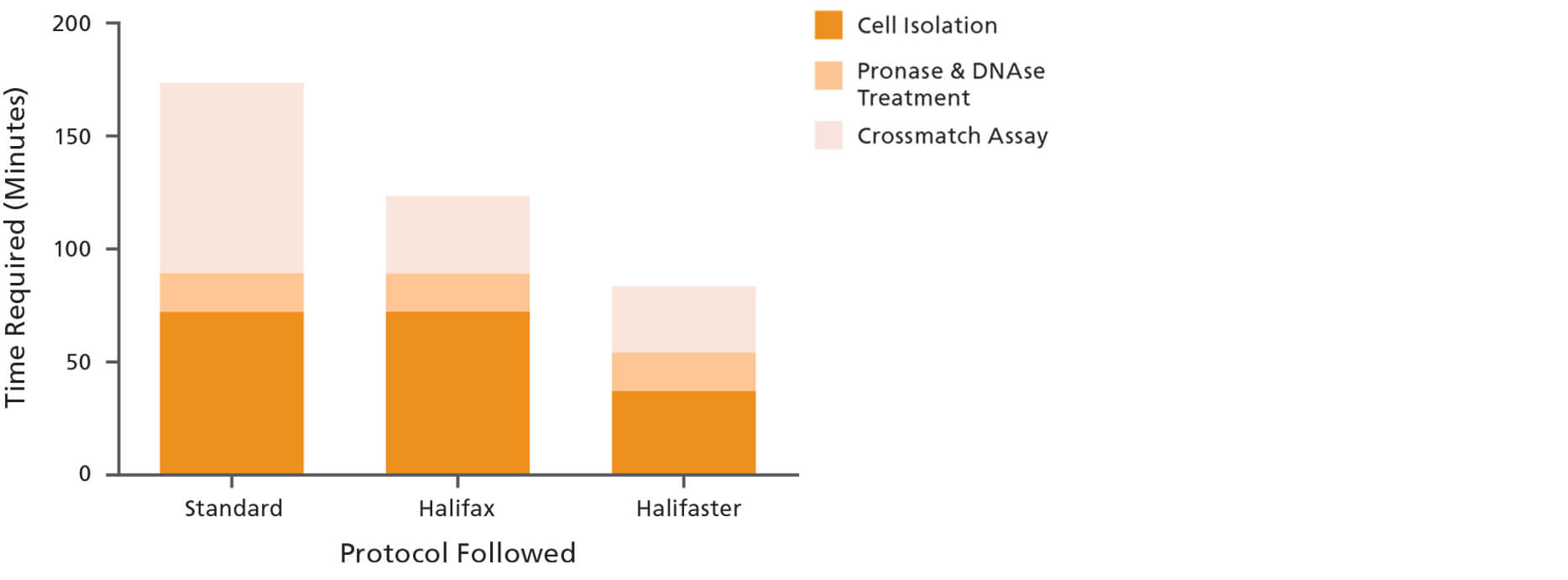
Figure 2. Following the Halifaster FCXM Protocol Reduces the Overall Time to Complete the FCXM Assay to Less Than 2 Hours
The cell preparation part consists of the donor lymphocyte isolation and treatment with pronase and DNAse. The Halifaster FCXM protocol reduced the time required for the cell preparation by almost 40% (from 90 to 55 minutes). The approximate total times it takes to complete the FCXM assay (including the cell preparation step) following the standard, Halifax and Halifaster FCXM protocols, correspond to 175 minutes, 125 minutes and 85 minutes, respectively. Figure taken from Development and Validation of a Rapid Optimized Flow Cytometry Crossmatch (FCXM) Assay
Improve purity while reducing the time of the cell isolation step
To isolate cells from blood and spleen samples, many laboratories use the density gradient centrifugation method. However, the purity of lymphocytes isolated using this method is variable and often poor, which may in turn affect crossmatch results.2 In addition, isolating cells using density gradient is very laborious and time-consuming—it can take up to 45 minutes to isolate cells from a single sample.
By adopting efficient cell isolation technologies you can reduce the time to obtain highly purified cells by almost 40% and even automate the process to increase sample throughput. This means faster and reliable crossmatch results you can feel confident about without compromising assay sensitivity.
Choose Reliable and Efficient Cell Isolation Technologies
Skip Centrifugation and Obtain Highly Purified Cells Faster
Isolating cells from samples using density gradient centrifugation is a very manual process that can easily introduce user variations. The centrifugation times may also need to be adjusted depending on the quality of the blood samples; older blood samples may have to be centrifuged for longer times and may require additional RBC lysis. The cell isolation step thus presents an opportunity for optimization and improvement to achieve faster, more reliable crossmatch assay results.
To address the issues associated with density gradient centrifugation, our scientists developed EasySep™ Direct technology. With an easy-to-follow protocol and a lower chance of introducing errors, cells can be isolated directly from blood, spleen, and lymph nodes in as little as 20 minutes using EasySep™ Direct. In addition, user variability and total hands-on technician time can be reduced by minimizing sample handling through automation, which can be achieved by combining EasySep™ Direct and RoboSep™ instruments.
How Does EasySep™ Direct Work?
EasySep™ Direct is a powerful immunomagnetic technology that isolates highly purified cells without centrifugation and RBC lysis. Cell isolations can be performed manually or can be automated using RoboSep™ instruments.
Implementation of the EasySep™ Direct cell purification had a very positive impact on our cell isolation and FCXM assay set-up time. Furthermore, we observed an improvement in events acquisition as well as time and ease of gating and analysis based on the improved lymphocyte purity.
Liwski RS et al. (2018)

Automating Cell Isolation with RoboSep™
A study presented during the ASHI 43rd Annual Meeting compared the EasySep Direct™ and RoboSep™ System with the traditional density gradient centrifugation method that uses Ficoll-Paque® to isolate total lymphocytes for the FCXM assay. The aim of the study was to improve efficiency and implement automated cell preparation.3 The authors concluded that the EasySep™ Direct and RoboSep™ system:
- Reduced the total isolation protocol time by 25%.
- Reduced total tech hands-on time by 55%.
- Increased purity of the cell preparations compared to Ficoll-Paque™.
… automation using EasySep™ [Direct] kit on RoboSep™ markedly improves the purity of the cells with identical results and sensitivity in the FCXM. The use of [EasySep™ Direct kit on RoboSep™] proved to be more efficient and cost effective, reducing not only the reagent cost but also more importantly “hands-on” tech time.
Fernandez-Bango C et al. (2017)
EasySep™ Direct for the Crossmatch Assay
EasySep™ Direct reagents are specifically designed to obtain highly purified lymphocytes that can be immediately used in the CDC crossmatch and FCXM assay. We are committed to the highest standard of quality and design controls, to provide reagents you can trust. As such, several EasySep™ Direct reagents are CE-marked as in vitro diagnostic devices in specific regions, including the European Union, Australia, and Canada
Table 1. EasySep™ Direct Products for Crossmatch Analysis
* Kit also works on other red blood cell containing samples (i.e. cord blood, buffy coat).
† This kit carries the CE mark and is available as a Class I In Vitro Diagnostic Device (IVD) in the European Union , Canada, Vietnam, Malaysia, Thailand and Australia. To learn more about the regulatory status of the product in your specific region, contact info@stemcell.com or visit Regulated Products at STEMCELL.
Explore these resources for HLA Labs
References
- Liwski RS et al. (2018) Rapid optimized flow cytometric crossmatch (FCXM) assays: The Halifax and Halifaster protocols. Hum Immunol 79(1): 28–38.
- Liwski R et al. (2016) P099 The impact of lymphocyte purity on flow cytometry crossmatch (FCXM) assay. It’s not purely theoretical. Hum Immunol 77, Supple: 110–111.
- Fernandez-Bango C et al. (2017) P230 Automation for flow cytometry crossmatch (FCXM) lymphocyte isolation using robosep. Hum Immunol 78: 224.