TeSR™-E7™ Episomal Protocol
.
- Document # 28065
- Version 1.0.2
- Mar 2019
Reprogramming Human Dermal Fibroblasts in TeSR™-E7™ to Induced Pluripotent Stem Cells Using an Episomal Vector System
The following protocol describes how dermal fibroblast cells can be reprogrammed to iPS cells using TeSR™-E7™ and an episomal vector system.
Equipment Required:
- Neon® Transfection System (Life Technologies Catalog #MPK5000).
- Neon® Transfection System 100 μL kit (Life Technologies Catalog #MPK10025)
Note: The method described here uses the Neon® Transfection System to transfect somatic cells with episomal vectors containing the reprogramming factors. Other vector systems or electroporation devices (e.g. Lonza Nucleofector™ or BioRad Gene Pulser Xcell™) may be used, but the protocol will need to be optimized for transfection efficiency and viability.
Reagents Required:
TeSR™-E7™ Reprogramming Medium
To make 500 mL TeSR™-E7™ Reprogramming Medium (Catalog #05914) combine:
- TeSR™-E7™/ReproTeSR™ Basal Medium (Catalog #05919, 480 mL)
- TeSR™-E7™ 25X Supplement (Catalog #05915, 20 mL)
Fibroblast Culture Medium
To make 500 mL Fibroblast Culture Medium, combine:
- DMEM (Catalog #36250, 440 mL)
- Fetal Bovine Serum (Catalog #06902/06952, 50 mL)
- MEM Non-Essential Amino Acid Solution (Catalog #07600, 5 mL
- L-glutamine (Catalog #07100, 5 mL)
Vectors Encoding Reprograming Factors
- Epi5™ Episomal iPSC Reprogramming Kit (Life Technologies Catalog #A15960)
Additional information regarding these episomal vectors available from Addgene:
- pCE-hOCT3/4 (Plasmid #41813)
- pCE-hSK (Plasmid #41814)
- pCE-hUL (Plasmid #41855)
- pCE-mp53DD (Plasmid #41856)
- pCXB-EBNA1 (Plasmid #41857)
Fibroblast Cells
- BJ Fibroblasts (ATCC® Catalog #CRL-2522™)
- Normal human dermal fibroblasts (NHDF; Lonza Catalog #CC-2511)
Note: Other fibroblast cell sources can be used, but transfection and reprogramming efficiencies may vary between cell lines. In general, fibroblasts will demonstrate higher reprogramming efficiencies at lower passage numbers (Figure 1).
Support Reagents
- D-PBS without Ca2+ and Mg2+ (Catalog #37350)
- Trypsin-EDTA (0.25%; Catalog #07901)
- Vitronectin XF™ (Catalog #07190) OR Corning® Matrigel® hESCqualified Matrix (Corning® Catalog #354277)
- mTeSR™1 (Catalog #85850) OR TeSR™-E8™ (Catalog #05990)
- DMEM/F-12 (Catalog #36254)
- Y-27632 (Catalog #72302/72304)
- Gentle Cell Dissociation Reagent (Catalog #07174)
Protocol:
Use sterile techniques when preparing all reagents and performing all aspects of the procedure.
1. Culturing Human Fibroblasts
Culture fibroblasts in Fibroblast Culture Medium at 37°C and 5% CO2. Replace culture medium every 2 - 4 days and passage with Trypsin-EDTA (0.25%) when the culture is approximately 85% confluent.
2. Matrix Coating of Cultureware
Successful reprogramming of fibroblasts in TeSR™-E7™ requires the use of a suitable matrix. Either Corning® Matrigel® hESC-qualified matrix (Matrigel®) or Vitronectin XF™ recombinant protein matrix can be used with TeSR™-E7™. Vitronectin XF™ is recommended if a defined and xeno-free culture system is desired. Cultureware should be pre-coated prior to transfection.
Procedures for coating cultureware with Matrigel® are outlined in the technical manuals ‘Maintenance of Human Pluripotent Stem Cells in mTeSR™1’ (Catalog #29106) and ‘Maintenance of Human Pluripotent Stem Cells in TeSR™-E8™’ (Catalog #29267). Procedures for coating with Vitronectin XF™ are outlined in the manual ‘Maintenance of Human Pluripotent Stem Cells in TeSR™-E8™’.
3. Preparation and Transfection of Fibroblasts
- To prepare fibroblasts for electroporation, remove culture medium and wash cells twice with D-PBS. Remove D-PBS.
- Add a sufficient volume of Trypsin-EDTA (0.25%) to cover the surface of the culture dish (e.g. add 3 mL Trypsin-EDTA if using a T-75 flask).
- Incubate at 37°C for 3 - 5 minutes, or until cells detach.
- When the cells are fully detached from the flask, add DMEM with 2% FBS at 1.5X the volume of Trypsin-EDTA to inactive the Trypsin (e.g. 4.5 mL for a T-75 flask). Transfer the solution to a 15 mL conical tube.
- Centrifuge for 5 minutes at 300 x g, remove supernatant, and resuspend cells in 5 mL D-PBS to wash.
- Count cells using Trypan Blue and a hemocytometer.
- Aliquot the appropriate volume of cell suspension containing 1 x 106 cells into a fresh conical tube, and centrifuge for 5 minutes at 300 x g. Remove supernatant and resuspend cells in the appropriate electroporation suspension buffer (for example, if using the Neon® Transfection System, resuspend the fibroblasts in 100 μL Resuspension Buffer R).
- Add 2µg of each episomal vector to the cell suspension and mix. Note: Amount of episomal vectors used may require optimization depending on transfection efficiency and fibroblast line.
- Electroporate cells with reprograming vectors according to the manufacturer’s instructions. We recommend using 1600 V, 10 ms pulse width, 3 pulses in a Neon® 100 µL pipette tip with the Neon® Transfection System.
- Transfer cells to a 15 mL conical tube containing 10 mL Fibroblast Culture Medium.
- Plate 50,000 cells/well (i.e. 0.5 mL cell suspension/well) onto Matrigel®-coated or Vitronectin XF™-coated wells of a 6-well plate. Note: Plating density may need to be optimized depending on growth kinetics of cells being reprogrammed.
- Add sufficient Fibroblast Culture Medium to each well (e.g. a final volume of 2 mL per well of a 6-well plate).
4. Reprogramming Induction Phase in TeSR™-E7™
- Two days (48 hours) after seeding transfected fibroblasts onto Vitronectin XF™-coated or Matrigel®-coated dishes, aspirate Fibroblast Culture Medium and replace with a sufficient volume of TeSR™ E7™ (e.g. 2 mL/well of a 6-well plate).
- Perform daily medium changes with TeSR™-E7™ until iPS colonies are ready to be manually isolated. This may take approximately 20 - 35 days. Note: During the first two weeks of reprogramming it is acceptable to double feed (i.e. add 4 mL medium/well) and skip a medium change the following day once per week. Note: The time to iPS colony emergence is cell line dependent. Plates should be monitored daily starting at day 15 for any new cell lines used.
5. Picking iPS Colonies and Transferring to TeSR™-E8™ or mTeSR™1
Between days 20 and 35, colonies resembling human embryonic stem cell colonies appear. Once colonies have reached a diameter of approximately 500 - 1000 μm, they can be manually isolated and cultured in maintenance medium such as mTeSR™1 or TeSR™ E8™. The procedures below should be performed under a stereomicroscope using sterile conditions.
- Add 2 mL/well of mTeSR™1 or TeSR™ E8™ to each well of a 6-well plate pre-coated with Matrigel® or Vitronectin XF™
Note: Y-27632 ROCK Inhibitor may be added to culture medium at a final concentration of 10 μM to improve survival of subcloned fragments. - Isolate the putative iPS cell colony using either a 22 Gauge needle or a pulled glass pipette to separate and scrape away any nonreprogrammed cells such as fibroblasts, partially reprogrammed cells, or differentiated cells.
- Cut the putative iPS colony into small fragments with the needle or pulled glass pipette.
- Using a micropipette with 200 μL filtered pipette tip, scrape colony fragments to loosen their attachment to the plate and aspirate into tip. Immediately transfer colony fragments to the dish prepared in step 5.1.
6. Culture of New iPS Cell Lines
For the first few passages we recommend manually passaging newly isolated iPS cell lines before adapting to chemical or enzymatic passaging. This can help reduce the presence of contaminating cells such as fibroblasts, partially reprogrammed cells, or differentiated cells. Once iPS cell lines are established, they can be chemically or enzymatically passaged as described in the technical manuals ‘Maintenance of Human Pluripotent Stem Cells in mTeSR™1’ (Catalog #29106) and ‘Maintenance of Human Pluripotent Stem Cells in TeSR™-E8™’ (Catalog #29267).
Expected Results:
TeSR™-E7™ has been used to reprogram adult normal human dermal fibroblasts (NHDF) and neonatal fibroblasts (BJ). We have observed reprogramming efficiencies that are consistent with Okita et al. (2011) for this vector system and are equivalent or greater than in KnockOut™ Serum Replacement- (Life Technologies) based iPS cell reprogramming medium.
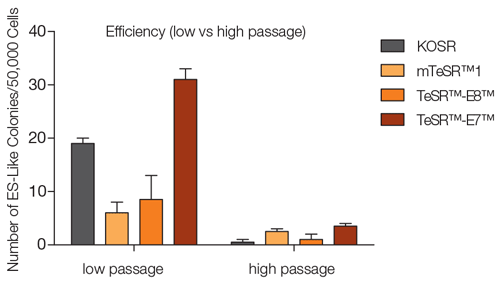
Figure 1. TeSR™-E7™ yields higher reprogramming efficiencies in feeder-free conditions compared to other media in both low and high passage NHDF
Low (p3) and high passage (p7) NHDF were reprogrammed as described in either TeSR™-E7™ or other media, and the number of ES-like colonies were counted after 28 days. TeSR™-E7™ yielded highest number of ES-like colonies in both high and low passage NHDF. In general, fibroblasts at lower passage number yield higher reprogramming efficiencies and are therfore recommended.
References:
- Okita K et al. (2011) A more efficient method to generate integration-free human iPS cells. Nat Methods 8(5): 409-12.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration

