Protocol for Genomic DNA Isolation from Mouse Tail/Animal Tissue or Cultured Cells
- Document # 27177
- Version 1.0.0
- Dec 2019
The following protocol is for genomic DNA isolation from cultured cells or animal tissue using the Genomic DNA Purification Kit (Catalog #79020). For complete instructions, refer to the Technical Manual (Document #10000005432).
Directions
A. Preparation of Cell Lysate
Prepare cell lysate from mouse tail or tissue, or from tissue culture cells, as indicated below.
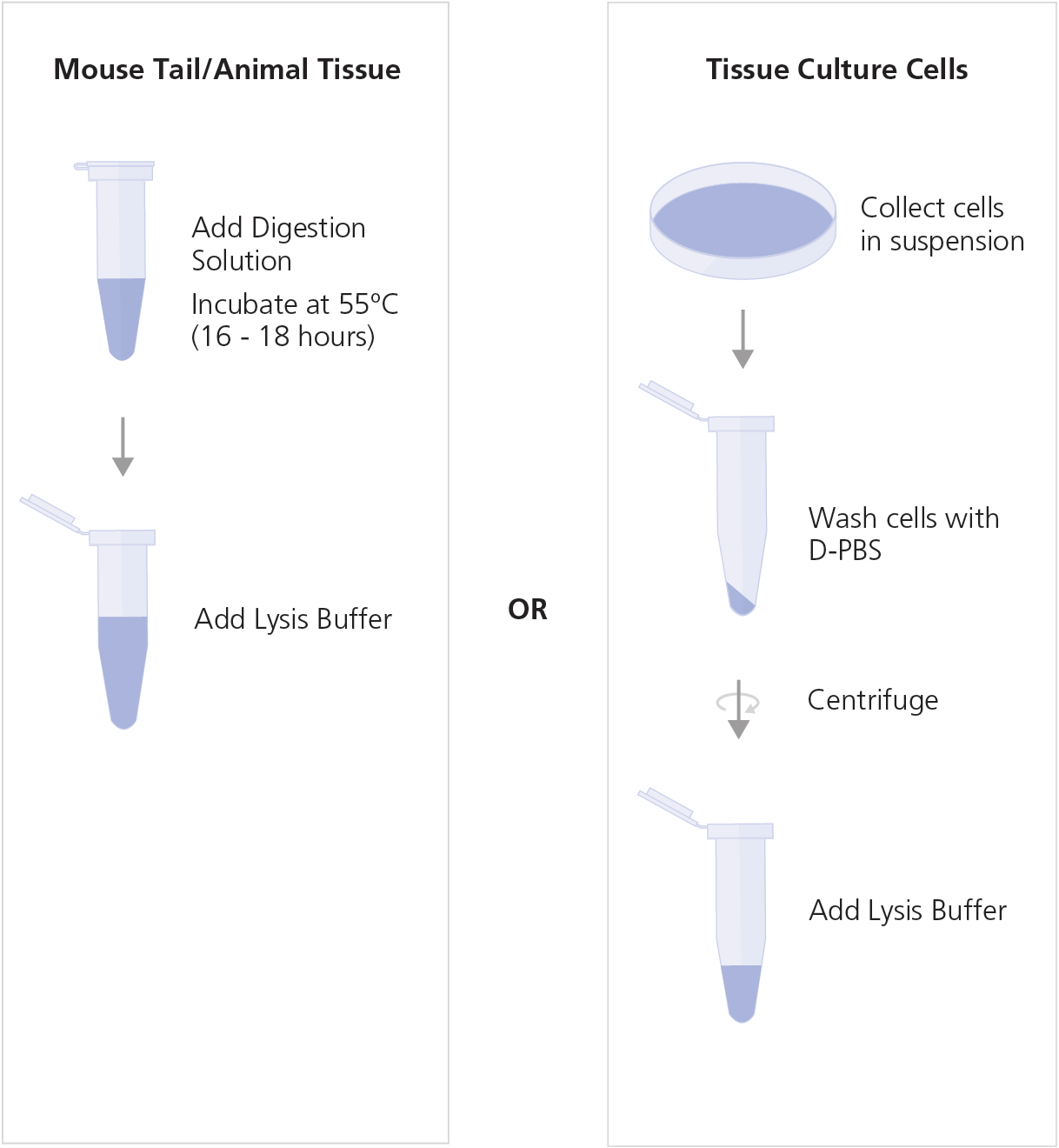
Mouse Tail or Animal Tissue Lysate
- Prepare Digestion Solution as indicated in Table 1. Mix thoroughly and store on ice.
- Cut a 0.5 - 1.2 cm length of mouse tail from the tip or weigh up to 20 mg of tissue sample in a clean DNase-free 1.7 mL microcentrifuge tube.
- Add 275 μL Digestion Solution to each tube.
- Incubate the sample tubes overnight (16 - 18 hours) in a 55ºC heating block or water bath.
- Add 250 μL Lysis Buffer to each sample. Vortex to mix.
- Proceed to DNA isolation.
Table 1. Preparation of Digestion Solution
Tissue Culture Cell Lysate from Cell Suspension
- Collect 1 x 104 to a maximum of 5 x 106 cells. Wash the cells once with D-PBS.
- Add 150 μL Lysis Buffer to the washed cells. Mix by pipetting up and down.
- Proceed to DNA isolation.
B. DNA Isolation
- Insert minicolumn into Collection Tube.
- Transfer lysate sample to the minicolumn assembly.
- Centrifuge at 13,000 x g for 3 minutes. Remove the minicolumn from the Collection Tube and discard the liquid. Reinsert the minicolumn in the Collection Tube.
- Add 650 μL Column Wash Buffer (with ethanol added). Centrifuge at 13,000 x g for 1 minute. Remove the minicolumn from the Collection Tube and discard the liquid. Reinsert the minicolumn in the Collection Tube.
- Repeat step 5 for a total of 4 washes.
- Empty the Collection Tube and place the minicolumn back in the tube. Centrifuge at 13,000 x g for 2 minutes to dry the membrane.
- Carefully transfer minicolumn to a new labeled 1.7 mL microcentrifuge tube.
- Add 250 μL nuclease-free water to the minicolumn. Incubate at room temperature for 1 - 2 minutes. Centrifuge at 13,000 x g for 1 minute. For mouse tail or animal tissue lysates, proceed to step 9. For tissue culture lysates, proceed to step 10.
- Add an additional 250 μL nuclease-free water to the minicolumn. Incubate at room temperature for 1 - 2 minutes. Centrifuge at 13,000 x g for 1 minute.
- Discard minicolumn and store purified DNA at -20ºC.
Genome Editing and Molecular Tools
Find products for genome editing and molecular biology, including the Total RNA Purification Kit and the Gel and PCR Clean-Up Kit.
Molecular Biology Methods Library
Explore more protocols, technical tips, and videos for various molecular biology techniques.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration