Protocol for Producing Monoclonal Cell Lines Using ClonaCell™ FLEX Semi-Solid Medium
.
- Document # 29298
- Version 1.0.1
- May 2023
Reduce the Time Needed to Isolate Producing, Monoclonal Cell Lines
Background
The protocol for semi-solid cloning in 96-well plates combines isolation of discrete colonies, each with a high probability of monoclonality, with the efficiency of selecting and expanding only producing clones. Conventional methods to select and clone mammalian cell lines involve multiple dilution steps in liquid medium. Semi-solid cloning using ClonaCell™ methylcellulose-based media from STEMCELL Technologies Inc. reduces the overall time necessary to produce monoclonal cultures by up to 19 days. ClonaCell™ FLEX is a semi-solid base medium that allows users to create a customized semi-solid medium by combining it with a 2X liquid culture medium appropriate for a specific cell type and process. ClonaCell™ FLEX is chemically defined and protein- and animal component-free. Using the standard protocol for selection and cloning of mammalian cells and hybridomas in ClonaCell™ media, cells (i.e. freshly transfected cells or fused myeloma cells and splenocytes) are suspended in selective semi-solid medium and incubated in 10 cm plates. Individual cells grow to form discrete, monoclonal colonies that are picked from the semi-solid medium after 10 - 14 days of incubation. Colonies are then transferred to individual wells of a 96-well plate and cultured in liquid medium prior to screening the supernatants for clones of interest.
The following technical note describes the use of ClonaCell™ FLEX customizable semi-solid base medium in 96-well plates to reduce the need to harvest and expand large numbers of colonies before screening (Figure 1). Cells suspended in ClonaCell™ FLEX are plated directly into wells of a 96-well plate. Cells grow in the semi-solid medium as discrete colonies and secrete protein products into the surrounding medium. Liquid medium is layered over the semi-solid medium and the secreted proteins diffuse into the liquid medium, which is then screened to identify colonies producing specific products. This method enables isolation of producing cultures with a high probability of monoclonality after only a single round of cloning. These combined benefits result in considerable time and labor savings.
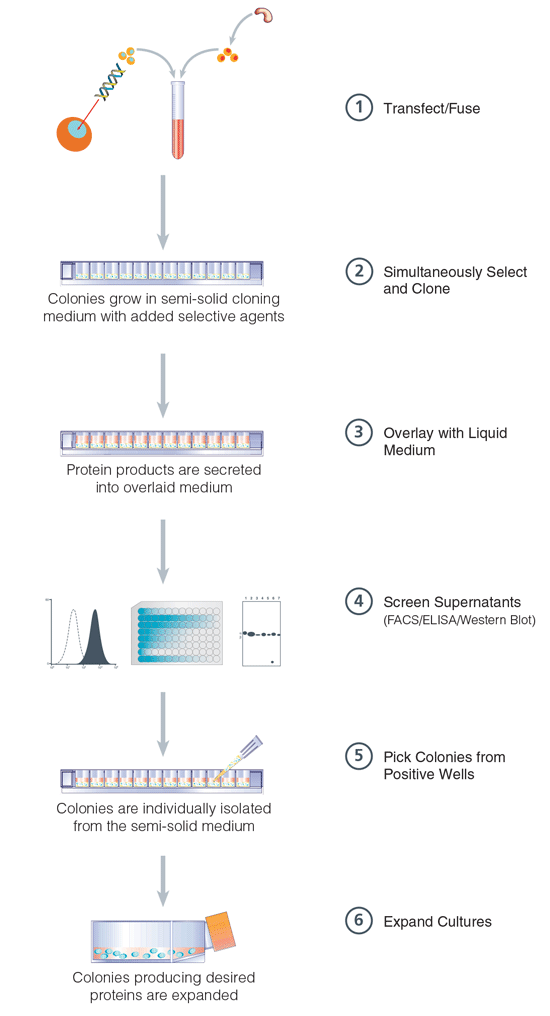
Figure 1. ClonaCell™ FLEX 96-Well Plate Procedure
Protocol
- On the day of transfection or fusion, place ClonaCell™ FLEX methylcellulose-based semi-solid base medium (Catalog #03818) at 2 - 8 °C to thaw overnight. Do not place medium in a water bath to thaw.
- Perform cell transfection or fusion of myeloma cells and splenocytes.
- Incubate the cells in liquid recovery medium without selective agents at 37°C in a humidified, 5% CO2 incubator for 16 - 24 hours.
- On the day after transfection or fusion, warm thawed ClonaCell™ FLEX semi-solid base medium to 37°C.
- Add 45 mL of 2X liquid growth medium concentrate to the 45 mL bottle of ClonaCell™ FLEX to make 90 mL of customized semi-solid growth medium. Add required growth and selection reagents in 1X liquid growth medium. The total volume of 1X medium containing additives and cells must not exceed 10 mL, such that the final volume of media, cells and additives is no greater than 100 mL. Shake vigorously to mix the contents of the bottle.
- Determine the optimal number of cells to plate per well to obtain one colony per well. If you already have experience with cloning by limiting dilution, plate the same number of cells per well in the semi-solid medium as you would in liquid medium. Plating at various cell densities is recommended as transfection or fusion efficiency may vary from experiment to experiment.
- Resuspend the cells in 1X liquid growth medium. It is critical that the volume of cells added in this step and the volume of reagents added in 1X medium in step 5 do not exceed 10 mL. This gives a final volume of 100 mL plated cell suspension from one 45 mL bottle of ClonaCell™ FLEX semi-solid base medium.
- Add the cell suspension in 1X liquid growth medium to the prepared semi-solid medium. Mix well and let sit for 15 minutes to allow the bubbles to rise to the surface.
- Using either a multi-channel pipettor and sterile wide-bore pipette tips, or a repeat pipettor and sterile syringe, dispense 60 - 80 μL of the cells in semi-solid medium into each well of a 96-well plate. This will yield between 12 - 16 plates depending on the volume plated. ClonaCell™ FLEX semi-solid medium is viscous and therefore difficult to pipette accurately; however, it is not critical to dispense exactly the same volume into each well.
- Incubate the plates at 37°C in a humidified, 5% CO2 incubator. The incubator should be well-humidified to prevent excessive evaporation. If desired, the plates may be placed inside a plastic container that allows proper gas exchange (e.g. 245 mm x 245 mm; Catalog #38039/100-0084) along with an uncovered Petri dish containing sterile water.
- Following 8 days of undisturbed incubation, examine wells for the presence of colonies by eye or microscope and gently overlay 150 μL of pre-warmed (37°C) liquid growth medium onto the semi-solid medium of each well that contains colonies. Alternatively, all wells may be overlaid with 150 μL of pre-warmed liquid medium, regardless of the presence of colonies, after which further analysis is performed on all wells.
- Incubate plates at 37°C in a humidified, 5% CO2 incubator for an additional 2 - 4 days. The overlay incubation time may be increased to ensure the detection of low-expressing clones.
- Carefully remove a maximum of 100 μL of the overlaid liquid medium without disturbing the colonies in the semi-solid medium. Test the supernatants for specific protein products using an appropriate assay system (e.g. ELISA, flow cytometry, Western blotting).
- Cultures in wells that test positive for desired protein products should be expanded by gently resuspending the contents and transferring them to a single well of a 24-well plate containing 1 mL of liquid growth medium. If a well contains more than one colony, it may be possible to harvest the clones separately using a pipette and transfer them to individual wells for expansion and retesting. If wells contain more than one colony and harvesting of individual colonies is not possible, the cultures need to be recloned either immediately after harvesting or after a 1 - 2 day recovery and expansion period in liquid medium. Recloning is not necessary for positive clones that can be harvested independently as these colonies should already be monoclonal. It is useful, however, to reclone these cultures when selecting for stable, high-producing subclones.
Time Comparison of Limiting Dilution Cloning, ClonaCell™ Semi-Solid Cloning, and ClonaCell™ 96-Well Semi-Solid Cloning Protocols
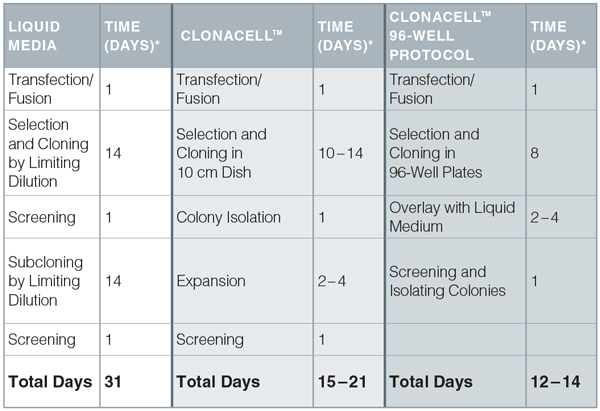
*Selection and cloning times may vary with different cell lines
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration