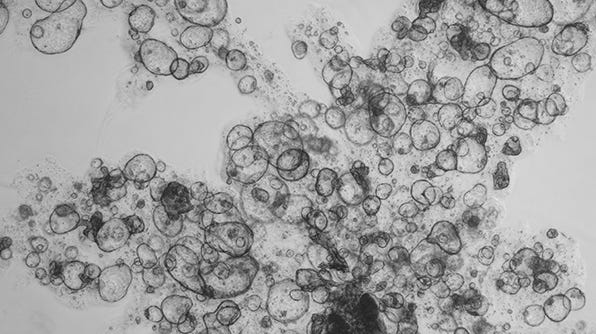
Expansion of Hepatic Organoids in Dilute Matrigel® Suspension Cultures Using HepatiCult™ Organoid Growth Medium
Growth in a dilute Matrigel® suspension allows the scale-up of cultures beyond what may be practical when plating in Matrigel® domes. Organoids grown in a dilute Matrigel® suspension are typically ready for passage earlier, usually within 4 - 7 days, compared to 7 - 10 days for dome cultures, and supports culture in larger, bioreactor formats (e.g. spinner flasks).
The below protocol describes the expansion of hepatic organoids in a dilute Matrigel® suspension culture using HepatiCult™ Organoid Growth Medium (Human).
Materials
- Healthy hepatic organoid cultures expanded in HepatiCult™ Organoid Growth Medium (Human)
- HepatiCult™ Organoid Growth Medium (Human) (Catalog #100-0385)
- DMEM/F-12 + 15 mM HEPES (Catalog #36254)
- 25% Bovine serum albumin (BSA) solution in water
- Corning® Matrigel® (Corning 356231, ≥ 8mg/mL protein)
- Antibiotics (e.g. gentamicin)
- 15 mL conical tubes (Catalog #38009)
- Ultra-Low Adherent (ULA) 6-Well Plate for Suspension Culture (Catalog #100-0083) OR 100 mL sterile spinner flasks
- Sterile 200 µL and 1000 µL tips
- 5 mL and 10 mL serological pipettes (Catalog #38003 and Catalog #38004)
- Styrofoam box with ice
- Incubator with atmospheric O2 and 5% CO2 at 37°C
- Orbital shaker or spinner flask-compatible stirring platform that can be placed in the incubator
Preparation
- Thaw and store Matrigel® on ice.
- Thaw HepatiCult™ Organoid Growth Supplement overnight at 2 - 8°C. Prepare 100 mL Matrigel®-supplemented HepatiCult™ Organoid Growth Medium (OGM + Matrigel®) on ice using sterile technique:
- 90 mL HepatiCult™ Organoid Basal Medium
- 5 mL HepatiCult™ Organoid Growth Supplement
- 5 mL Matrigel®
- Add antibiotics (e.g. 50 µg/mL gentamicin)
- Mix thoroughly. Store on ice.
- Prepare 50 mL of serum-supplemented DMEM/F-12 + 15 mM HEPES (DMEM + BSA):
- 2 mL 25% BSA solution in water (1% final concentration)
- 47.9 mL DMEM/F-12 + 15 mM HEPES
- Mix thoroughly. Store on ice.
- If using ULA 6-well plates, place the plates at 2 - 8°C for at least 10 minutes. If using spinner flasks, place flasks on ice for at least 10 minutes before use.
Note: Once thawed, use HepatiCult™ Organoid Growth Supplement immediately or aliquot and store at -20°C. After thawing aliquots, use immediately. Do not re-freeze.
Note: Prepare OGM + Matrigel® fresh for every use. Do not store any remaining volumes long-term.
Protocol
- Process organoids based on the culture format in use as follows:
If passaging from a 6-well suspension culture:
- Using a serological pipette, transfer the contents of all wells to be passaged to a 15 mL conical tube. Leave tubes at room temperature for 5 - 10 minutes to allow organoids to settle.
- Remove and discard as much of the spent medium as possible without aspirating any organoids, leaving up to 0.5 mL of medium in the tube.
- Add 1 mL cold DMEM + BSA to the tube and proceed to step 2.
If passaging from dome cultures:- Check that the Matrigel® domes to be passaged are intact (i.e. the whole dome remains attached to the plate and no loose Matrigel® pieces or organoids are observed in the well). If the dome is intact, proceed to the next step. If the dome is loose, add cold DMEM + BSA to top up the total volume in the well to 1 mL and let sit for 1 minute, then skip the next step and proceed without aspirating the medium in these wells.
- Without touching the dome, aspirate and discard the medium in each well to be passaged.
- Using a 1 mL pipettor, forcefully add 1 mL cold DMEM + BSA to the center of each dome, then let sit for 1 minute. Proceed to step 2.
- Using a 1 mL pipette tip on the pipettor, vigorously pipette the total volume up and down 45 times, taking care not to generate bubbles.
Note: This results in mechanical breakdown of organoids and Matrigel® into fragments between 30 - 100 μm in size. If many larger fragments remain, continue pipetting the suspension until the majority of fragments are < 100 μm in size.
Note: Cell strainers can be used to collect a suspension of more uniformly sized fragments if desired.
- Determine the volume required to seed the desired number of fragments in the subsequent passage. Transfer this volume to a 15 mL conical tube containing 1 mL of DMEM + BSA.
Note: Seeding density should be optimized for each donor. ~1000 - 3000 fragments per mL of OGM + Matrigel® is recommended.
- Centrifuge tubes from step 3 at 290 x g for 5 minutes.
- Aspirate as much of the supernatant as possible without disturbing the pellets (the pellet is often not visible).
- Resuspend pellet as described below and store tube on ice.
- 0.5 mL OGM + Matrigel® per well to be seeded if using a 6-well plate.
- 3 mL OGM + Matrigel® if seeding spinner flask.
- Place the pre-cooled 6-well plate or spinner flask on ice in the biosafety cabinet. Add 1.5 mL cold OGM + Matrigel® per well of the 6-well plate or 47 mL cold OGM + Matrigel® to the spinner flask.
- Add the OGM + Matrigel® + fragment volume from step 6 to the culture vessel, swirling the vessel to mix.
- Carefully transfer the plate or flask to an orbital shaker set to 80 rpm or stirring platform set to 33 rpm respectively, in an incubator at 37°C and 5% CO2.
- Perform a full-medium change every 2 - 3 days as follows:
- Tilt the culture vessel at a ~45 degree angle and allow organoids to settle for 3 - 5 minutes. If using a spinner flask, be careful not to disturb the central arm, as this will stir the organoids back into suspension.
- Remove and discard ~75% of the spent medium, taking care not to aspirate any organoids.
- Add an equivalent volume of fresh, room temperature complete HepatiCult™ OGM.
Note: OGM does not need to be supplemented with Matrigel® for medium changes. To avoid weekend medium changes, perform medium changes on Mondays, Wednesdays, and Fridays. Hepatic organoids should be passaged before the lumen turns dark and collapses, approximately every 4 - 7 days.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration