Mesenchymal Stromal Cells: Markers, Isolation and Culture, Differentiation, and Therapeutic Potential
The bone marrow (BM) stroma contains a heterogeneous population of cells, including endothelial cells, fibroblasts, adipocytes and osteogenic cells, and it was initially thought to function primarily as a structural framework upon which hematopoiesis occurs
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
- Document # 29018
- Version 4.0.0
- Jun 2015
MSCs in the Bone Marrow Stroma
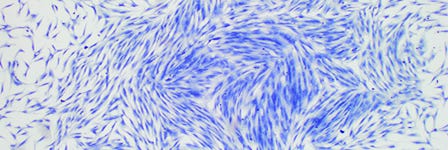
The bone marrow (BM) stroma contains a heterogeneous population of cells, including endothelial cells, fibroblasts, adipocytes and osteogenic cells, and it was initially thought to function primarily as a structural framework upon which hematopoiesis occurs.1 Later evidence demonstrated, however, that at least two distinct stem cell populations reside in the bone marrow of many mammalian species: hematopoietic stem cells (HSCs) and mesenchymal stromal cells (also known as mesenchymal stem cells; MSCs), with the latter responsible for the maintenance of the non-hematopoietic bone marrow cells. MSCs, also termed multipotent marrow stromal cells or mesenchymal stromal cells, are a heterogeneous population of plastic-adherent, fibroblast-like cells, which can self-renew and differentiate into bone, adipose and cartilage tissue in culture.2-5 In the late 1960s, Friedenstein and colleagues established that single cell suspensions of BM stroma could generate colonies of adherent fibroblast-like cells in vitro. These colony-forming unitfibroblasts (CFU-Fs) were capable of osteogenic differentiation and provided the first evidence of a clonogenic precursor for cells of the bone lineage.6 The CFU-F assay is now widely used as a functional method to quantify stromal progenitors.7,8 Functional in vitro characterization of the stromal compartment by Dexter et al. in the 1970s then revealed its importance in regulating the proliferation, differentiation and survival of HSCs.1 CFU-F initiating cells in vivo have been shown to be quiescent, existing at a low frequency in human bone marrow.9
Isolation, Marker Specificity and Functional Properties of MSCs
Although MSCs are traditionally isolated from bone marrow, cells with MSC-like characteristics have been isolated from a variety of fetal, neonatal and adult tissues, including cord blood, peripheral blood, fetal liver and lung, adipose tissue, compact bone, dental pulp, dermis, human islet, adult brain, skeletal muscle, amniotic fluid, synovium, and the circulatory system.10-18 Accumulating evidence indicates a perivascular location for these MSC-like cells in all tissues, implying that all MSCs are pericytes19 that closely encircle endothelial cells in capillaries and microvessels in multiple organs.19-26 Pericytes are thought to stabilize blood vessels, contribute to tissue homeostasis under physiological conditions, and play an active role in response to focal tissue injury through the release of bioactive molecules with trophic and immunomodulatory properties.25 Pericytes and adventitial cells also natively express mesenchymal markers and share similar gene expression profiles as well as developmental and differentiation potential with mesenchymal cells.27-29 Pericytes may represent a subpopulation of the total pool of assayable MSCs at least within the bone marrow. An extensive study by Crisan and colleagues has established further links between MSCs and pericytes by validating the phenotype of pericytes as CD146+, NG2+, PDGFR+, ALP+, CD34-, CD45-, vWF- and CD144- throughout human fetal and adult organs.26 Despite their shared markers and perivascular location in vivo, more evidence is required to prove that MSC-like cells in every tissue are derived from or indeed function as pericytes.
In an attempt to standardize the definition of an MSC, the International Society for Cellular Therapy (ISCT) proposed the concept of essential minimal criteria for MSCs in culture. The four minimal defining criteria for MSCs are: i) adherence to plastic under standard tissue culture conditions ii) expression of CD105, CD73, CD90 iii) lack of expression of CD45, CD34, CD14/CD11b, CD79/CD19 and HLA-DR surface markers and iv) differentiation into adipocytes, osteoblasts and chondroblasts in vitro.30 Nevertheless, there is no consensus regarding the MSC phenotype, because of the broad variety of potential tissue sources and the differences in cell isolation and cell culture procedures used. In addition, differences in media formulations (FBS, platelet lysates, growth factor combinations), plating density and oxygen tension may affect the gene profile, epigenomic state and phenotype of the mesenchymal population.31
Human MSCs
MSCs are reported to express SH2 (CD105), SH3/SH4 (CD73), CD29, CD44, CD90, CD71, CD106, CD166, STRO-1, GD2, and CD146.4,24,25,32-38 The current ISCT criteria are limited to the definition of human BMderived MSCs and may not be applicable to MSCs derived from other tissues (e.g. the negative markers are primarily specific for hematopoietic cells, which comprise the vast majority of the marrow and are the major contaminating cells in BM-derived stromal cultures, and this may not be true for MSC cultures derived from other tissues). For example, Traktuev and colleagues identified a multipotent CD34+ population derived from the adipose stromal vascular fraction which shared pericyte and MSC surface markers.39 This is supported by a publication referring to the stromal vascular fraction (SVF) of the adipose tissue and the adipose tissue-derived stromal cells as a population identified phenotypically as CD45-CD235a-CD31-CD34+ (a statement under the joint authorities of the International Federation of Adipose Therapeutics and Sciences (IFATS) and the ISCT.40 The expression of CD34 by adipose-derived stromal and stem cells (ASC) observed at the time of isolation from primary adipose tissue may then be lost following extensive culture in vitro. Further characterization revealed that ASCs expressed markers in common with other mesenchymal stromal/stem cells populations including CD90, CD73, CD105, and CD44 and remained negative for CD45 and CD31. ASC can be distinguished from BM-derived MSCs by their positivity for CD36 and lack of CD106.
Similar to expression of CD34 on freshly isolated ASC, we and others44-46 have evidence that freshly isolated BM-MSCs express low levels of the CD45 antigen. Once in culture, BM-MSCs rapidly lose CD45 expression, making the use of CD45 as a negative selection marker for isolating MSCs from fresh BM inefficient. The inability to phenotypically distinguish between MSCs of differing potency hinders the ability to identify more primitive from mature cells and precludes the analysis of different subpopulation of MSCs in culture.
Moreover, the proposed positive markers for MSCs do not necessarily correlate with MSC function, particularly when related to their differentiation potential in vitro. In culture, CD73, CD105 and CD90 continued to be highly expressed on human BM-derived MSCs from early to late passages (P3 to P9) when the same cell population showed significant reduction in their differentiation potential after passage 5, particularly when cultured in serumcontaining medium (unpublished data).
To date, researchers have reported poor correlation between the broad differentiation potential of the MSCs in vitro and the function of these cells in vivo. For example, adipose-MSCs were reported to exhibit inferior osteogenic potential to that of bone marrow-MSCs in vitro, however the in vivo studies were more controversial.47 It is possible that MSC populations derived from different primary tissues may have intrinsic differences in their capacity to differentiate into the various mesenchymal lineages, despite exhibiting identical phenotypes in vitro. More rigorous investigation of the properties of MSC from various tissues is needed to further elucidate the differences in potency of cells which, despite appearing phenotypically indistinguishable, may in fact be functionally very different.
As no single unique marker for MSC isolation has been reported, the isolation of human MSCs is thus heavily reliant on their ability to adhere to and subsequently proliferate on tissue culture plastic. Culture selection is often used in combination with Ficoll™ separation and/or pre-enrichment using various cocktails of antibodies.44 Enriched populations of MSCs have been isolated from human BM aspirates using a STRO-1 monoclonal antibody in conjunction with antibodies against VCAM-1/CD106,32 CD271,45 D7-Fib46 and CD49a.47 Other molecules co-expressed by CD271+ MSCs include PDGFR-α HER-2/ErbB2 (CD340) and frizzled-9 (CD349).48 However, not all cells expressing these markers are MSCs.
A recent publication identified a set of biophysical markers predictive of a multi-potent MSC subpopulation in cultures derived from fetal and adult BM. The three biophysical markers included small cell diameter, low cell stiffness and high nuclear membrane fluctuations and, together, were able to identify multipotent stem cells from committed osteochondral progenitors.49
Mouse MSCs
Various phenotypes for mouse MSCs have been proposed, with little or no overlap with human MSC phenotypes. Short and Simmons’ extensive study of mouse CFU-F enrichment identified the femoral compact bone as a richer source of progenitor cells than the marrow plug within it. By performing the CFU-F assay on single cell suspensions depleted of hematopoietic cells, this group reported a CFU-F frequency of 2689 ± 58 CFU-F/106 cells in compact bone, compared to 102 ± 80 CFU-F/106 cells in mouse BM.50 They then used multiparameter fluorescence-activated cell sorting (FACS) to identify a subpopulation of proposed stromal (mesenchymal) precursors with the composite phenotype Lin-CD45-CD31-SCA1+.51 However, an alternate population of primitive mesenchymal cells derived from adult mouse BM that express stage-specific embryonic antigen-1 (SSEA-1) was characterized by Bonnet’s group.52 The SSEA- 1+ population demonstrated extensive differentiation potential, forming astrocyte-, endothelial- and hepatocyte-like cells in vitro, and was found in the putative mesenchymal compartment in vivo, comprising about 0.04% of the total Lin-/CD45-/CD31- fraction of adult mouse BM.52 In addition, Suire and colleagues defined another phenotype for BM-derived mouse MSCs obtained from enzymatically digested marrow plugs at a frequency two orders of magnitude higher than observed in BM harvested by conventional methods.53 These cells are found in the stromal vascular-fraction and may be isolated prospectively using the composite phenotype Lin-PDGFRαβ+.
Oxygen Concentration and MSC Culture
The phenotype of MSCs varies not only between tissues but also between species and, in addition, may be affected by culture conditions. Differences in cell surface phenotype were reported between a population of human MSCs (hMSCs) cultured at 21% O2 as defined by Pittenger et al.,4 and the Marrow Isolated Adult Multilineage Inducible (MIAMI) cells cultured in low oxygen tension as described by D’Ippolito et al.41 The MIAMI cells were shown to have increased expression of primitive cell markers such as SSEA-4 when cultured at low pO2 compared to the same cells cultured at 21% pO2. At 3% O2 MIAMI cells also have upregulated levels of mRNAs for OCT-4, REX-1 and HIF1-alpha, whereas MSCs as defined by Pittinger do not express these primitive markers.4,42 Comparative analysis of the proteomic profile of MSCs, MIAMI cells and human embryonic stem cells (hESCs) reveals a closer relationship between hMSCs and MIAMI cells, however, MIAMI cells have more proteins in common with hESCs than with hMSCs.43
Like many other cell types,54-58 mouse MSCs demonstrate enhanced proliferation and optimal clonogenicity when cultured in a hypoxic environment (Brenton Short, unpublished data).51 It has also been reported that increased proliferation of rat bone marrow-derived MSCs at 5% oxygen is most likely due to increased expression of hypoxia inducible factor (HIF), which in turn upregulates genes involved in metabolism, cell proliferation and survival.58, 59 Culture in low oxygen conditions thus appears to be a critical factor in the in vitro expansion of mouse mesenchymal cells.
How to Create a Hypoxic Environment for Cell Culture
Learn how to mimic physiological conditions to support MSC growth in vitro with a Hypoxia Incubator Chamber.
Assays to Define MSCs
Unlike the HSC field where multiple rigorous assays are available (e.g. NOD-SCID, LTC-IC, CFC) to analyze the different function and maturation stage of distinct stem and progenitor cells of HSCs, the MSC field is lacking stringent assays to demonstrate self-renewal and multi-potency derived from single cells and stemness properties through serial transplantation experiments in vivo. Most researchers currently characterize a heterogenous pool of MSCs derived from high plating density cultures which do not accurately reflect cell behavior at a clonal level. More stringent studies utilizing clonally derived MSC populations may enable the identification of sub-populations of cells better able to maintain the characteristics required of a stem cell population. Similarly, these potency assays could help increase the efficacy and safety of cell therapies utilizing large numbers of culture-expanded cells. Generation of gene and protein expression databases of MSCs from diverse MSC tissue, donor and culture conditions may provide knowledge of the heterogeneity of MSC subpopulations (FDA Voice, 2014). Transplantations of heterotopic ossicles can serve as an assay to show the intrinsic capacity of cells to generate specific tissue in animals reflecting the functional capacity of MSCs in vivo.60 Should serial transplantation of MSCs derived from a primary ossicle prove to be feasible and reproducible, the question of MSC self-renewal may finally be answered. The use of animal models is essential to assess the efficacy of MSCs in vivo and together with rigorous in vitro assays, the true nature and function of the MSC may finally be unraveled.
Differentiation Potential of MSCs
Cultured MSCs can differentiate into mesodermal cell types (e.g. adipocytes, osteoblasts, chondrocytes) under specific stimuli. However, accumulating evidence has revealed that the tissue of origin impacts the differentiation potential of MSCs.60,61 The stem cell niche could be a determining factor for stem cell self-renewal and lineage differentiation potential. Isolation of MSCs from a particular tissue can yield distinct subset of MSC population with diverse differentiation potential. MSClike cells derived from synovium were shown to be superior for chondrogenic potential compared to MSCs derived from bone marrow tissues.62 This information is relevant when designing a therapy to stimulate resident progenitors for repair in articular cartilage. Another study comparing BM-MSCs to dental pulp- MSCs reported less differentiation ability into adipogenic lineage but stronger differentiation into osteogenic lineage of dental-pulp –MSCs compared to BM-MSCs.63 The apparent ability of MSCs to give rise to cells of multiple germ layers in culture must be examined with care, as undifferentiated mesenchymal cells have been shown to spontaneously express neural64 as well as smooth muscle cell markers in culture.65 The effect of tissue-specific origin of MSCs on their differentiation potential into specific lineage needs to be taken into consideration for cell therapy application.
Therapeutic Potential of MSCs
The mechanisms by which mesenchymal cells repair damaged tissues in vivo is still poorly understood. Recent evidence suggests that repair is achieved using paracrine factors released by mesenchymal cells, rather than by the transdifferentiation of mesenchymal cells into specific tissue cell types. Paracrine secretion by MSCs has been reported to support tissue repair by promoting neovascularization and increasing angiogenesis. For example, exosome purified from culture medium conditioned by human ESC-derived MSCs was recently identified as the active compound for reducing infarct size in pig and mouse models of myocardial ischemia.66 Other ongoing studies examining the efficacy of transplanted mesenchymal cells in animal models of myocardial infarction,67 lung injury,68 kidney damage69 and neurological diseases70 may provide further insight into mechanisms underlying MSC-mediated tissue repair. Understanding the biology and the role of different mesenchymal cell subpopulations in tissue repair will be key to determining their potential for various therapeutic applications. In recent years, over 400 clinical trials worldwide have used MSCs to treat various diseases (www.clinicaltrials.gov).71 Most trials are currently in Phase I/II for various diseases such as heart disease, diabetes, cancer, bone/cartilage, neurological and immune-related disorders. MSCs are attractive candidates for cell therapy, being: 1) easy to isolate and expand in culture, 2) able to home to sites of inflammation, 3) able to differentiate into multiple mesodermal cell types, 4) immunomodulatory, 5) low in immunogenicity, and 6) able to confer cytoprotection by secreting a broad spectrum of cytokines and growth factors.
Bone repair
Devine et al. first showed that cultured mesenchymal cells could home to the bone marrow in non-human primates.72 One study has also shown that culture-expanded MSCs can persist and contribute to de novo bone formation in vivo. Eight weeks after MSCs were placed into a porous cylinder and implanted into a rat femur, the implant-containing defect healed completely.73,74 However, culture-expanded MSCs are unable to home to osteogenic sites. Two explored methods for overcoming this challenge are: 1) peptidomimetic ligands for ß1 integrin on the MSC surface, coupled to bisphosphonate to facilitate migration to the bone surface,75 and 2) RNAi against Ckip-1, a negative regulator of osteogenesis that targets RunX2 for degradation,76 to bone surfaces using AspSerSer6 liposomal targeting moieties. The latter method was the first to provide a means of facilitating bone formation without concomitant osteoclast activation and bone resorption.
Cultured allogeneic human mesenchymal cells have also been used in clinical trials for the treatment of children suffering from osteogenesis imperfecta.77 The first year after MSC engraftment, patients showed improvement as measured by reduced incidence of bone breakages, however, these effects declined with time. The decline could have been caused by senescence of the cultureexpanded cells or by terminal differentiation during cell culture and passaging,78 which may be related to epigenetic changes of mesenchymal cells during prolonged culture.79
Bone/Cartilage: Cellular allografts containing MSCs have been used in high risk foot and ankle surgery for bone reconstruction purposes, resulting in improved healing and a reduced interval to partial weight-bearing.80 A serial transplantation experiment using fluorescently tagged MSCs showed MSCs were able to localize to areas of bone injury regardless of their administration route.81 MSCs are also reported to promote cartilage repair in rabbit models of cartilage defect using electrospun nanofiber based technology.82
Muscle
Mulitpotent human muscle resident MSCs (hmrMSCs) were recently characterized as population of cells expressing CD73+CD105+ and CD90- isolated from human skeletal muscle tissue.83 The contribution of non-myogenic progenitor cells in the regeneration and production of abnormal tissue formation in heterotopic ossification (HO), a human skeletal muscle disorder, was explored. Primary human skeletal muscle tissues from control and HO patients were digested with collagenase, and the resulting cells were then expanded in xeno-free medium before being clonally selected based on CD90 expression. Adipogenic, osteogenic and chondrogenic differentiation potential of the cells was also evaluated. The hmrMSCs (CD90-) population showed robust differentiation into adipocytes, osteogenic cells, chondrocytes and also into brown adipocytes expressing uncoupling protein 1 (UCP1). This is the first report to show the presence of UCP1-positive adipocytes in human HO and to confirm that the hmrMSCs from adult skeletal muscle can give rise to all cell lineages present in HO.
HSC engraftment
MSCs appear to be extremely sensitive to chemical and radiation-induced damage, and remain at a significantly low frequency after exposure.84 Transplantation may perturb the ability of MSCs to regulate hematopoietic cells, which would explain the slow and skewed recovery of many immune cell populations.85 Co-transplanting MSCs with HSCs could thus enhance long-term HSC engraftment, as has been demonstrated by in utero co-transplantation of fetal sheep with human bone marrow stromal cells and human HSCs.86 MSCs may also prevent the onset of immune cell-induced graft-versus-host disease (GVHD) following transplantation,87 as cultured MSCs do not express MHC-class II antigens on their cell surface and can suppress a primary mixed lymphocyte reaction. When MSCs cultured in both FBS- and platelet lysate-based media were given to patients with chronic and acute GVHD, half of the patients responded positively, with pediatric patients faring best.88 In one clinical trial, the infusion of MSCs into eight patients with steroidrefractory grade III - IV acute GVHD even resulted in the complete disappearance of GVHD in six of eight patients.89
Immunomodulatory Effect of MSCs
One property that greatly increases the value of MSCs in therapeutic applications is their ability to modulate immune responses. MSCs can exert their immunomodulatory function by releasing inhibitory factors, stimulatory factors or the expression of other surface molecules depending on the microenvironment. MSCs can escape the immune system because BM-MSCs are not recognized by NK cells as they lack expression of HLA Class I surface markers. They also lack expression of HLA Class II antigens, which is desirable for translplantion applications.
The immunosuppressive activity of MSCs is poorly understood but recent reports provide some mechanistic insights into key regulatory molecules. Programmed death-ligand 1 (PD-L1)/ CD274 also known as B7 Homolog 1 (B7-H1) has been shown to be expressed in cultured MSCs and is strongly upregulated following IFN-γ stimulation. Combination therapy using rapamycin and MSCs induced immune tolerance to allografts, but monoclonal antibodies against B7-H1 were shown to abrogate this tolerance leading to allograft rejection.90 The immunomodulatory effects of MSCs were shown to be mediated in part through upregulation of regulatory immune cells including CD4+CD25+FoxP3+ T cells and tolorogenic dendritic cells and a decrease in alloantibody levels. MSCs that expressed B7H1 may also induce the apoptosis of activated T-cells as co-culture of CD4+CD25- T cells with MSCs resulted in significant upregulation of programmed cell death-1 receptor (PD-1) on activated T cells.91 Similar results were reported by Chinnadurai who further examined the role of IFN-γ in the ‘licensing’ of MSCs to inhibit the proliferation of activated T cells.92 Both MSCs and IFN-γ licensed MSCs inhibited T-cell proliferation, however only IFN-γ licensed MSCs significantly inhibited Th1 cytokine (IFN-γ, TNFα and IL-2) production as well as T-cell degranulation. This IFN-γ licensed MSC inhibitory effect on T-cells is thought to be dependent on indoleamine 2,3-dioxygenase (IDO), however Chinnadurai showed that MSC IDO catalytic function is dispensible with regard to MSC driven T-cell inhibition and identified the B7-H1 PD1 pathways as essential effectors in blocking T-cell function. Further complexity was also suggested by a recent report that IFN-γ treatment of MSC upregulated HLA-DR /Class II MHC after 48 hours and MSCs ability to inhibit T cells through B7-H1 was dependent upon the presence of HLA-DR.93
A novel mechanism for MSC induced immunosuppression was recently proposed by Obermajer and colleagues who showed that cells of the Th17 type, predominantly associated with the rejection of allogeneic solid organ grafts, can be directly converted into a regulatory T cell (Treg) type.94 The induction of Tregs was preceded by development of a CD11b(hi)Gr1(int) myeloid-derived immunosuppressive cell mediated Th17. They identified retinoic acid receptor-related orphan receptor γ as a common factor in the differentiation of Treg and Th17 cells. The identification of specific subset of T cells IL-17A+Foxp3+ double-positive and ex-IL-17- producing IL-17A-Foxp3+ in this paper argues for direct conversion as the mechanism for MSC-mediated immunotolerance. This proposed mechamism where MSC-induced myeloid-derived immunosuppressive cells act as mediator for immunetolerance without complete immunosuppression may have significant implication for therapeutic application.
The importance of species variations related to immunosuppression mechanisms by MSCs are sometimes overlooked. For example, immunosuppression by human-derived MSCs is mediated by indoleamine 2,3-dioxygenase (IDO), whereas mouse MSCs is mediated by nitric oxide. When the expression of IDO and inducible nitric oxide synthase (iNOS) were examined in human and mouse MSCs after stimulation with their respective inflammatory cytokines, human MSCs expressed extremely high levels of IDO, and very low levels of iNOS, whereas mouse MSCs expressed abundant iNOS and very little IDO.95 Thus, studies of MSC-mediated immunomodulation in mice may not be informative in the setting of human disease.
Despite the extensive use of MSCs in clinics and in many ongoing clinical trials, there is a lack of long term safety data examining the use of MSCs in humans. It has been reported that MSCs may variously exert an anti- or pro-tumor growth effect depending on the tumor type and its microenvironment.96-98 Tumor formation in patients receiving MSCs has not been reported to date, however the risk of potential tumorigenicity when MSCs are used in therapy was recently discussed.97 Two possible scenarios include 1) malignant transformation of the MSCs (possibly as a result of extensive proliferation in vitro and resultant accumulation of genetic pertubations) and 2) the immunosuppressive effect of MSCs which could enhance the growth of existing malignant cells of a patient. The absence of a suitable in vivo model system which can completely rule out the risk of tumor formation is of concern. The potential of MSCs to transform regenerative medicine is undeniable, from repair of skeletal maladies, to the treatment of GVHD, to their efficacy in modulating the immune system and abrogating the severity of myocardial infarcts. Despite the challenges facing the field, MSCs may one day be able to treat a broad range of debilitating conditions. Through rigorous studies and the development of novel assays this potential may soon be realized.
Explore More Resources
Reference
- Dexter TM, et al. J Cell Physiol 91: 335-344, 1977
- Bruder SP, et al. J Cell Biochem 64: 278-294, 1997
- Mackay AM, et al. Tissue Engin 4: 415-428, 1998
- Pittenger MF, et al. Science 284: 143-147, 1999
- Horwitz EM, et al. Cytotherapy 7: 393-395, 2005
- Friedenstein AJ. Haematol Blood Transfus 25: 19-29, 1980
- Friedenstein AJ, et al. Cell Tissue Kinet 3: 393-403, 1970
- Clarke E, et al. Bone Marrow Transplant 4: 596-597, 1989
- Epikhina S, et al. Biull Eksp Biol Med 81: 55-57, 1976
- Campagnoli C, et al. Blood 98: 2396-2402, 2001
- In’t Anker PS, et al. Blood 102: 1548-1549, 2003
- Zuk PA, et al. Mol Biol Cell 13: 4279-4295, 2002
- Erices A, et al. Br J Haematol 109: 235-242, 2000
- De Bari C, et al. Arthritis Rheum 44: 1928-1942, 2001
- Kuznetsov SA, et al. J Cell Biol 153: 1133-1140, 2001
- Tondreau T, et al. Stem Cells 23: 1105-1112, 2005
- Carlotti F, et al. Islets 2: 164-173, 2010
- Gesine P, et al. PLoS One 7(4): e35577, 2012
- Caplan AI. Cell Stem Cell 3: 229-230, 2008
- Andreeva ER, et al. Tissue Cell 30: 127-135, 1998
- Doherty MJ, et al. J Bone Miner Res 13: 828-838, 1998
- Bianco P, et al. Stem Cells 19: 180-192, 2001
- Zannettino AC, et al. J Cell Physiol 214: 413-421, 2008
- Shi S, et al. Bone Miner Res 18: 696-704, 2003
- Sacchetti B, et al. Cell 131: 324-336, 2007
- Crisan M, et al. Cell Stem Cell 3: 301-313, 2008
- Corselli M, et al. Arterioscler Thromb Vasc Biol 30: 1104-1109, 2010
- Feng J, et al. Expert Opin Biol Ther 10: 1441-1451, 2010
- Crisan M, et al. Organogenesis 7: 101-104, 2011
- Dominici M, et al. Cytotherapy 8: 315-317, 2006
- Roobrouck VD et al. Stem Cells 4: 583-589, 2011
- Simmons PJ, et al. Blood 78: 55-62, 1991
- Gronthos S, et al. J Cell Sci 116: 1827-1835, 2003
- Martinez C, et al. Blood 109: 4245-4248, 2007
- Haynesworth SE, et al. Bone 13: 69-80, 1992
- Galmiche MC, et al. Blood 82: 66-76, 1993
- Conget PA, et al. J Cell Physiol 181: 67-73, 1991
- Sordi V, et al. Blood 106: 419-427, 2005
- Traktuev et al. Circ Res. 102: 77-85 2008
- Bourin P. Cytotherapy 15: 641-648, 2013
- D’Ippolito G, et al. J Cell Sci 117: 2971-2981, 2004
- D’Ippolito G, et al. Bone 39: 513-522, 2006
- Roche S et al. Int. J Pharm 440: 72-82, 2013
- Clarke E, et al. Blood 98: 355a, 2001
- Quirici N, et al. Exp Hematol 30: 783-791, 2002
- Jones EA, et al. Arthritis Rheum 46: 3349-3360, 2002
- Deschaseaux F, et al. Br J Haematol 122: 506-517, 2003
- Buhring HJ, et al. Ann N Y Acad Sci 1106: 262-271, 2007
- Wong Chen Lee, et al. PNAS 111: E4409-18. doi: 10.1073, 2014
- Short BJ, et al. Blood 100: 62a, 2002
- Short BJ, et al. Arch Med Res 34: 565-571, 2003
- Anjos-Afonso F, et al. Blood 109: 1298-1306, 2007
- Suire C, et al. Blood 119: e86-95, 2012
- Studer L, et al. J Neuroscience 20: 7377-7383, 2000
- Csete M, et al. J Cell Physiol 189: 189-196, 2001
- Reykdal S, et al. Exp Hematol 27: 441-450, 1999
- Csete M, Ann N Y Acad Sci 1049: 1-8, 2005
- Lennon DP, et al. J Cell Physiol 187: 345-355, 2001
- Ohnishi S, et al. Stem Cells 25: 1166-1177, 2007
- Bianco P, et al. Nat Med 19: 35-42, 2013
- Bianco P, et al. Cell Stem Cells 2: 313-319, 2009
- Liu H, et al. J Biomed Mater Res A (2014) doi: 10.1002/jbm.a.35303
- Ponnaiyan D and Jegadeesan V, Eur J Dent 8: 307-313, 2014
- Deng J, et al. Stem Cells 24: 1054-1064, 2006
- Drost AC, et al. Ann N Y Acad Sci 1176: 135-144, 2009
- Lai RC, et al. Regen Med 6: 481-492, 2011
- Minguell JJ, et al. Exp Biol Med (Maywood) 231: 39-49, 2006
- Ortiz LA, et al. Proc Natl Acad Sci USA 99: 2199-2204, 2002
- Kunter U, et al. JAM Soc Nephrol 17: 2202-2212, 2006
- Phinney DG, et al. Curr Pharm Des 11: 1255-1265, 2005
- Trounson A, et al. BMC Med 9:52 2011
- Devine SM, et al. Exp Hematol 29: 244-255, 2001
- Kadiyala S, et al. Tissue Engin 3: 173-185, 1997
- Bruder SP, et al. J Orthop Res 16: 155-162, 1998
- Guan M, et al. Nat Med 18: 456-462, 2012
- Zhang G, et al. Nat Med 18: 307-314, 2012
- Horwitz EM, et al. Nat Med 5: 309-313, 1999
- Banfi A, et al. Exp Hematol 28: 707-715, 2000
- Shibata KR, et al. Stem Cells 25: 2371-2382, 2007
- Scott R and Hyer CF. Foot and Ankle Surgery 52: 32-35, 2013
- Lin P, et al. Molecular Therapy, 22: 160-168. 2014
- Shafiee A, et al. J Biomed Mater Res A 99: 467-478, 2011
- Downey J, et al. Bone 71: 164-170, 2015
- Galotto M, et al. Exp Hematol 27: 1460-1466, 1999
- Witherspoon RP, et al. Semin Hematol 21: 2-10, 1984
- Almeida-Porada G, et al. Blood 95: 3620-3627, 2000
- Tse WT, et al. Cytotherapy 3: 417a, 2001
- Ringden O and Leblanc K. Best Pract Res Clin Haematol 24: 65-72, 2011
- Ringden O, et al. Transplantation 81: 1390-1397, 2007
- Wang H, et al. Transpl Immunol 31: 65-74, 2014
- Yan Z, et al. Immunol Lett 162: 248-255, 2014
- Chinnadurai R, et al. J Immunol 192: 1491-1501, 2014
- Jang IK, et al. Transplant Proc 46: 1638-1641, 2014
- Obermajer N, et al. J Immunol 193: 4988-4999, 2014
- Ling W, et al. Cancer Res 74: 1576-1587, 2014
- Guan J and Chen J. BioMed Rep 1: 517-521, 2013
- Barkholt L, et al. Cytotherapy 12: 753-759, 2013
- Bruno S, et al. Front Immunology 5: 382-392, 2014