Tips for Immunofluorescence Imaging, Featuring the Engevik Sisters

In this Q&A, Drs. Mindy, Amy, and Kristen Engevik offer tips and tricks for perfecting your immunofluorescence (IF) imaging and describe how their passion for STEM led each of them to pursue a career in research. Mindy and Amy are both Assistant Professors at the Medical University of South Carolina (MUSC) in the department of Regenerative Medicine and Cell Biology for digestive disease research, while Kristen is currently a Postdoctoral Associate at Baylor College of Medicine in the Department of Molecular Virology & Microbiology. The #SisterLab trio are well-known in the IF imaging sphere; they are published in high-impact journals, avid #FluorescenceFriday and #MicroscopyMonday participants, and keen contestants in STEMCELL’s annual #StemCellfie contest. You can see more of their stunning images on Twitter (@AmyEngevik, @MicroMindy and @thekengevik) and Instagram (@the_engevik_labs), or hear more from them in their Immunology Podcast episode.
Published March 2023
The Q&A interview has been lightly edited for clarity and brevity.
Immunofluorescence (IF) Imaging Tips and Tricks
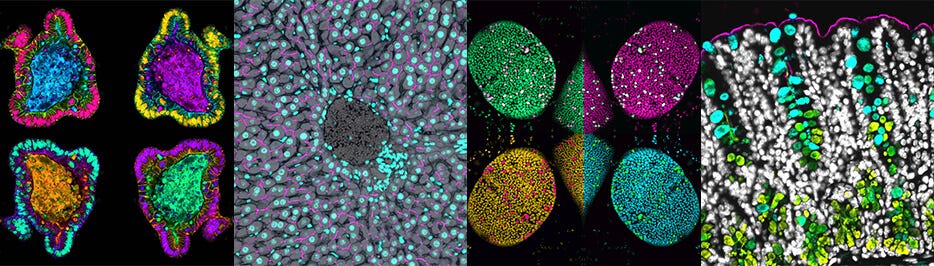
Mouse small intestinal tissue stained for villin (white), Muc2 (blue) and E-cadherin (magenta). Nuclei were stained with Hoechst and are in yellow/green.
Do you have any tips for choosing antibodies?
Mindy: The first thing I look at is the company’s information (antibody datasheet). I look very closely at their stained images and make sure that the staining they show is in the correct location (i.e., staining the right cells, in the right spot of the cell, not binding to non-specific locations, etc.). I also look up publications that have used the antibodies and double check that the staining looks specific. I always try to find antibodies that have been validated with knockout experiments (either in mice or in cell lines). I also like to check the human protein atlas to check what cell types the protein is normally expressed in and compare my staining to ensure that my staining pattern matches and I don’t have non-specific signals. When I stain tissue or cells, I like to combine antibodies generated from different species to highlight different cellular structures.
I find that primary antibodies are the most important part of staining, and making sure your antibody is binding to the right ligand is well worth your time. I find that most secondary antibodies are high quality and don’t have a lot of variability.
Dr. Mindy Engevik
How do you choose appropriate controls?
Mindy: For new antibodies, I always stain tissue samples I know should harbor the protein and those that shouldn’t. When possible, I love to validate the antibodies using knockout tissue. If knockout tissue isn’t accessible, I perform immunostaining using only secondary antibodies to compare to staining of primary and secondary antibodies. If the staining doesn’t match the expected localization of my protein of interest, I will use a peptide to block the staining and then use the primary and secondary antibody to see if the staining is non-specific.
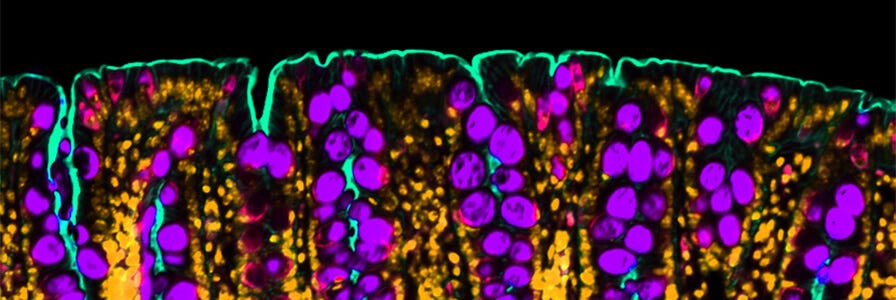
Mouse colon stained for Muc2 (purple) to identify goblet cells and gamma actin (teal) to label the apical membrane. Nuclei are in orange.
Do you have any tips for fixing tissues?
Amy: In my opinion, tissue fixing is one of the most crucial steps for getting good immunofluorescence images. If you start with poorly fixed or over-fixed tissue you will have an uphill battle to get acceptable images, no matter how good your antibodies or protocols are. I generally fix tissue (stomach, intestine, colon, liver) for 18 hours in either 4% paraformaldehyde solution (PFA) or 10% neutral buffered formalin at 4°C. Make sure that you orient or finely slice (for example with liver) the tissue before fixing. For the stomach and intestine, I use Whatman™ paper to lay the tissue flat and spread it out. I then fix the tissue on the Whatman™ paper so that when I roll the tissue after fixation, the tissue is flat and makes beautiful swiss rolls, which can be stored easily before cutting.
What’s the best way to optimize antibody dilution and cell density?
Amy: For new antibodies, I try to follow the manufacturer’s suggestions for antibody dilution specifically for immunofluorescence. Often, the datasheets merely state “dilution is assay dependent”. In that case, I generally start with a low dilution to make sure that the antibody works (1:100). Usually, 1:100 works well, but I increase or decrease the dilution as needed. I find that the majority of antibodies that I use require a dilution of 1:200. If I can’t get a signal from an antibody, I either try a different fixative (like glyoxal), change the antigen retrieval buffer, or use frozen sections instead of formalin-fixed, paraffin-embedded (FFPE).
Live Imaging
What are your top tips for staining in preparation for live imaging?
Kristen: If you’re using any form of fluorescent dye for imaging, it’s important to thoroughly wash your sample before and after adding your dye—otherwise you run the risk of an extremely noisy background and difficulty properly focusing on your cells. It’s also important to recognize that cells have a time limit for which they will tolerate dyes and that some dyes (like Hoechst33342 for staining the nuclei during live imaging) will eventually cause cell death after a few hours. If you can transduce your cells to express your biosensor of interest, I’d highly recommend using that method over fluorescent dyes.
Imaging Specific Tissue Types
What tips do you have for imaging host-viral interactions?
Kristen: I recommend using reporter viruses that have a fluorescent tag to track where the viruses are and what cells they are infecting. It’s also helpful to image cells that form confluent monolayers and can be fluorescently tagged by biosensor dyes or transduced. Also, for long-term imaging, it’s very important to check the focus at least an hour or so after initially starting your imaging run, to ensure the cells are still in focus and haven’t moved. It’s also helpful to pre-heat the microscope stage at least one hour before starting any live imaging.
For staining paraffin-embedded tissues: “Use a pressure cooker for heat-mediated antigen retrieval, and use primary antibodies that have a high specificity for your epitope of interest.”
Dr. Amy Engevik
Are there any special considerations for imaging calcium signaling?
Kristen: I recommend using genetically encoded calcium sensors (such as GcAMP6S) rather than using a dye-based approach (such as fluo-4). The dyes load differently depending on the cells being used and it can be tricky to ensure that you are detecting calcium fluxes in diverse cell lines without a biosensor.
Creating Publication-Worthy Images
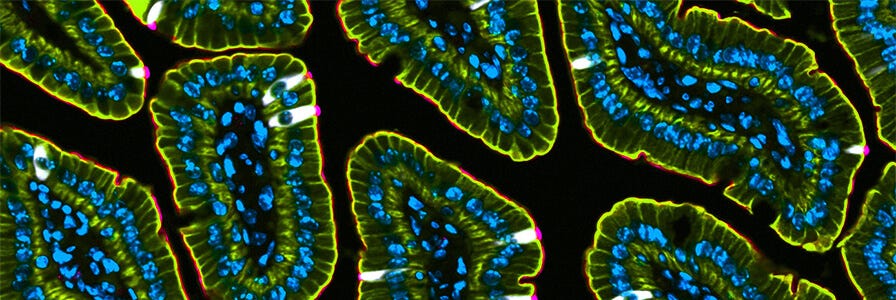
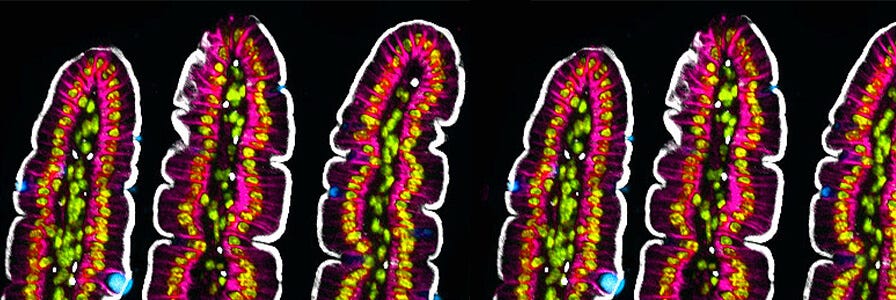
A cross section of intestinal villi from a mouse. Tuft cells were labeled using a DCLK1 antibody (white). The apical membrane was stained with villin and is visualized in pink. Pan cytokeratin staining is seen in yellow/green. Nuclei are seen in blue.
How do you process your images for publications?
Mindy: I usually export images as single grayscale TIFF files and then merge the files using Adobe Photoshop. For image analysis, I like to use ImageJ and the many plug-ins that are available in the software. The look-up tables (LUTs) are also great in ImageJ. For single-stain images, I like to invert the grayscale images to better visualize the staining. I use ImageJ to stack images for z stacks. I usually perform image stitching using the confocal microscope software.
Building a Career in Research
What inspired you each to pursue gut science?
Mindy: In a way, we fell into the gut by chance. Growing up, we were all pretty curious and interested in science. As the oldest, I was the first to get into science, and after finishing my Masters in Microbiology, I started working in a lab that focused on the gut. I decided to pursue a PhD in a gastrointestinal (GI)-centric lab and I found my passion for gut research and the microbiome.
Amy: My PhD work was my first exposure to gut science. I studied pathways that regulated gastric ulcer repair. I really enjoyed the research I did during my PhD. Interestingly, all of us (Mindy, Kristen, and I) joined different labs during our PhDs that had GI research programs.
Kristen: When Mindy first started working in a lab, I was an undergraduate student and Mindy told me about summer research internships. For the next three summers, I worked in the same lab as Mindy and became really interested in the gut. That was what initially sparked my interest in the GI tract.
I think that one of the reasons we all ended up pursuing GI research is that we could all offer unique perspectives on different aspects of the gastrointestinal tract. In a way, it provided innate collaborations between all of us where we could offer suggestions or feedback on how to approach different GI research questions.
Dr. Amy Engevik
Overcoming Barriers as Women In STEM
What Are Current Barriers for #WomenInSTEM and How Do You Believe They Can Be Overcome?
Amy: I think that the current system is not designed for women to succeed. We have all heard of the “leaky pipeline” where women are well-represented in graduate school for biological sciences but are highly under-represented at the faculty level. I think inherent gender bias and the demanding workload of scientific careers during biological windows for women starting families are the main culprits. I think that team-based science will create better pathways for women in academia. As we are learning how integrated various systems are, which are traditionally studied in isolation (i.e., gut-brain, gut-liver, gut-lung, gut-heart axes), team-based science seems to be the best way to advance our understanding and to drive scientific discovery. I also think that team-based science will provide greater oversight of scientific reproducibility. I have benefited enormously from having Mindy and Kristen’s expertise, insight, and collaboration. I recognize that this isn’t common, but I hope that in the future there will be female lab leaders that work in team settings to collectively run diverse scientific research programs. The ability to share workload, especially when there are unexpected health or life circumstances, would benefit men and women.
Microaggressions. Microaggressions are the everyday, subtle behaviors or words that convey bias. It can be as simple as people referring to male colleagues as doctor x and to female colleagues by solely their first name. But over time, these microaggressions chip away at people. We are all human beings who are prone to mistakes and I think the important thing is to have difficult conversations and make people aware of how their actions are perceived. I think we all can do better by being more intentional with our behavior.
Dr. Mindy Engevik
Kristen: While I do think it’s slowly starting to change, I think another barrier is having strong women role models and mentors across STEM. Thankfully, more effort is being made to recognize the contributions women have had in major scientific discoveries and advances in history. But with the high under-representation of women faculty in many departments/fields, it can be difficult to find women mentors or sponsors in higher positions to provide feedback, support, or who can help advocate on your behalf to help advance your career to higher professorial positions. I think it also highlights the need to build more support networks that can help overcome the challenges of a career in STEM.
Career Advice for Graduate Students
What's Your Best Advice for Grad Students Hoping to Pursue a Career in Academia?
Mindy: Work hard. I think that success in science is a lot about hard work. Much of what we do as scientists isn’t intuitive and can involve acquiring difficult skills. Practice and hard work help you to gain the skills that will enable you to succeed in academia.
Amy: Build resilience. Academia is a difficult career path that results in daily rejection. If you want to succeed you will have to learn to be resilient. I would also advise grad students to build meaningful relationships with their peers. If you succeed in securing a tenure track position, someday your peers will be reviewing your grants, manuscripts, and award applications. It will benefit you to have left good impressions with the people who may be deciding your level of success.
Establish your network and don’t be afraid to approach leaders in your field. Getting to know your scientific peers and establishing relationships with principal investigators can help you make crucial next steps in your career. Having a wide network is especially helpful in finding postdoctoral positions and in securing funding. Individuals recognizing your name or your work goes a long way.
Dr. Kristen Engevik
Downloadable Resources
For more tips to help with your #CareerInSTEM, and for conference and networking toolkits and checklists, browse our collection of downloadable content.
Lab Organization Templates and Spreadsheets
Maximize your efficiency in the lab with this collection of templates and spreadsheets.
Networking and Conference Toolkit
Ensure you’re prepared for your next conference with these networking tips, presentation do's and don'ts, LinkedIn best practices, and more.
Journal Club Toolkit
Efficiently run a journal club with these resources including an attendance sheet template, presentation checklist, feedback form template, and more.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration