Intestinal Organoids
Intestinal Organoids
Intestinal organoid cultures are three-dimensional (3D) in vitro tissue models that incorporate many of the physiologically relevant features of the in vivo intestinal tissue. These features include a polarized epithelial layer surrounding a functional lumen and all of the cell types of the intestinal epithelium present in proportions and relative spatial arrangement that recapitulate what is observed in vivo.
Within the last decade there has been a dramatic shift in the availability of tools and model systems used to study the intestinal epithelium with the development and adoption of intestinal organoid cultures occupying a central position within this movement. Since the introduction of the mouse small intestinal organoid model in 2009,1 there has been an avalanche of developments in this field, including development of culture conditions for human organoids derived from primary colonic tissue,2 as well as from human pluripotent stem cells (hPSCs).3 Various experimental techniques have also been developed in parallel with, and applied to, intestinal organoid cultures with a scientifically synergistic effect. Some of these techniques include novel tools for genetic manipulation,4,5 approaches for in vitro disease modelling6–9 and innovative co-culture system with autologous cell types10,11 or bacteria,12–14 as well as viral infection models.15,16 The development of such techniques as applied to intestinal organoid cultures has vastly increased the utility of this model system for a wide variety of purposes. While much of the initial groundwork for the organoid culture system originally sprang from deep roots in developmental biology, the maturing model system is now being applied to a wide variety of research fields, including upstream drug discovery and patient-specific drug screening, cancer and immunology studies, and the pathogenesis of infectious agents. The number of researchers adopting intestinal organoid culture systems to enrich their specific research programs is rapidly expanding in both the basic research and medical communities.
The Intestinal Epithelium
The intestinal epithelium incorporates several distinct cell populations, including the rapidly dividing intestinal stem cells (ISCs) that facilitate the typical four-to-five day turnover cycle of the adult intestinal epithelium.17 This property of rapid regeneration at intestinal stasis makes the intestine a uniquely convenient model system for epithelial cell biology and adult stem cell biology studies both inside and outside the specific context of intestinal function.
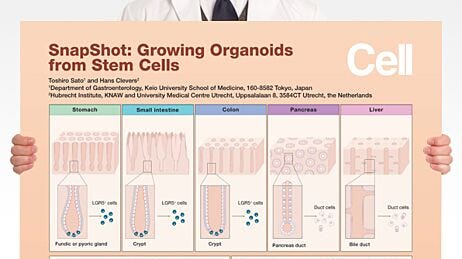
The adult intestinal epithelium is primarily composed of six cell types that are arranged in a crypt-villus structure18 (Figure 1). At the base of the intestinal crypt, the ISCs are found intercalated with Paneth cells,17 credited with much of the signaling required to maintain the ISC niche. Transit amplifying cells are partially differentiated cells that migrate upward via a physical crypt exclusion mechanism as the ISCs below them divide. As these cells move upward out of the crypt, they move along signaling gradients that trigger them to differentiate, giving rise to the mature cell types that populate the villus domain. Mature cells include enterocytes, which make up the majority of the villus epithelium and carry out nutrient absorption; goblet cells, which secrete mucus to protect the epithelial lining and help move intestinal contents through the lumen; and enteroendocrine cells, which respond to chemical actuators in the luminal contents by secreting hormones into the body to maintain nutrient metabolism.
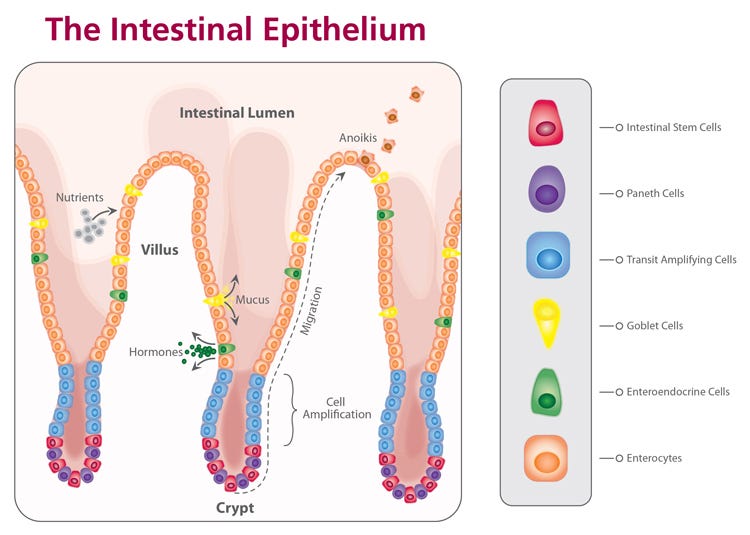
Figure 1. Diagram of the small intestinal epithelium highlighting the identity and spatial arrangement of key epithelial cell types.
The LGR5+ stem cells retain their capacity to self-renew and regenerate the intestinal epithelium primarily due to their position within a stem cell niche.2 The intestinal niche has been well-characterized, and shown to consist of spatial gradients of high WNT and Epithelial Growth Factor (EGF), while Bone Morphogenetic Protein (BMP) signals are inhibited. Understanding of these niche signals heavily informed development of the culture conditions for intestinal organoids.
Types of Intestinal Organoids
Organoids Derived from Primary Intestinal Tissue
The seminal work on the intestinal organoid culture system that came out of the laboratory of Hans Clevers in 20091 describes a culture system wherein the stem cell niche of the adult intestinal stem cells is recapitulated in vitro. This allows for establishment of organotypic intestinal epithelial cell cultures that maintain the regenerative properties of the in vivo intestine. These intestinal organoids, sometimes referred to as enteroids, are propagated from epithelial intestinal stem cells that exist within the adult intestinal tissue, and as such form an isolated epithelial structure in culture. This model allows interrogation of the intestinal epithelial system and direct manipulation of stem cell niche signaling without the confounding influence of the associated mesenchyme.
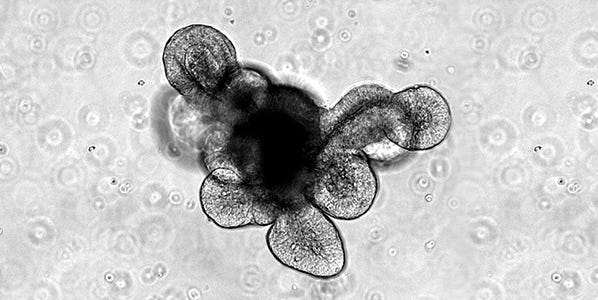
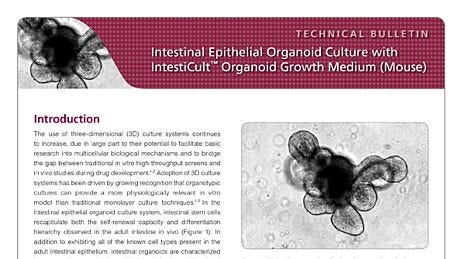
The first protocol published by Sato et al.1 described the isolation of intact intestinal crypts from mouse intestinal tissue and their subsequent culture to form organoids. Intestinal crypts are embedded in a dome of Matrigel® extracellular matrix and submerged in a growth medium that contains specific growth factors designed to mimic the signaling present at the intestinal crypt base in vivo. This work demonstrated that generation of intestinal organoids was possible from single, sorted LGR5+ intestinal stem cells derived from the dissociation of intestinal crypts.1 When cultured using this method, the organoids form an epithelial monolayer surrounding a central lumen, as well as budding crypt domains which are reported to contain the intestinal stem cells and Paneth cells that constitute their niche. Corresponding to the in vivo intestinal epithelium, the cells that make up the organoid epithelium are extruded and sloughed off into the lumen of the organoid. This results in the collection of cellular debris within the lumenal compartment over time and the associated decline in viability of the cultures, even in the presence of appropriate growth factors. The organoid cultures are therefore passaged periodically by dissociating the organoids from the Matrigel® and breaking them into fragments which are reseeded in new cultures. This process can be repeated indefinitely with remarkable genetic stability,19 and constitutes an effective expansion method for the intestinal stem cell population.
Subsequent to the establishment of this culture system for mouse small intestine, it was adapted to allow for generation of organoids from human small intestine, and human and mouse colonic crypts and single intestinal stem cells.2,20 While the organoids derived from intestinal and colonic crypts share many characteristics, they also exhibit significant differences. These are reflected in the cellular dynamics of the in vivo tissues, as well as the response of the tissue-specific adult stem cells to the specific niche signal-concentrations present in the cell culture medium. Typically, while mouse small intestinal organoid cultures tend to exhibit extensive budding of crypt-like domains, colonic organoids derived from both mouse and human crypts tend to exhibit a more cyst-like morphology with either significantly less pronounced or absent budding. Similarly, there is an increased requirement for WNT signaling when culturing human organoids compared with mouse.2 This drives elevated stem cell proliferation and results in a more cystic phenotype for human gut organoid cultures compared with the corresponding mouse organoids.
There have been many advancements and modifications to the basic culture methodology first developed for mouse and human intestinal epithelial organoids. These subsequent studies have introduced methodologies for specifically adapting the culture system for patient tumor-derived tissues with different growth factor dependencies21,22; growth of organoids in formats appropriate for medium- to high-throughput compound screening23,24; and use of defined extracellular matrix systems,25 among other application-specific adaptations.
Organoids Derived from Pluripotent Stem Cells
Soon after the introduction of the intestinal stem cell-derived organoid culture system, a methodology for culturing human intestinal organoids derived from human pluripotent stem cells (hPSCs) was published that used much of the same methodology for 3D organoid culture.3 In contrast to the intestinal epithelial organoids grown from isolated crypts or LGR5+ intestinal stem cells, intestinal organoids derived from differentiated hPSCs incorporate a mesenchymal compartment that contributes to the intestinal stem cell niche signaling present in the cultures.18 Intestinal organoids derived from directed differentiation of hPSCs also exhibit characteristics reminiscent of the longer-term processes that take place during normal tissue development; at early passages the organoids are characterized by a distinctly fetal intestinal phenotype whereas full maturation of the intestinal epithelial cells is thus far only achievable through transplantation of the organoids into mouse kidney capsules.26 Recapitulation of intestinal development in the hPSC-derived organoid system makes this model an excellent in vitro tool for examining intestinal developmental milestones in an easy-to-manipulate experimental system.18 Additionally, this system enables generation of organoids from individual patients without the requirement of intestinal or colonic biopsy, making it a useful tool for investigating the phenotypic properties of the intestinal epithelium from individuals with a wide range of genetic characteristics.
The protocol for establishing intestinal organoids from undifferentiated cells encompasses differentiation of hPSCs to definitive endoderm and initiation of mid/hindgut specification as monolayer culture, through intestinal fate induction in 3D culture as intestinal organoids.3 Interestingly, the formation of 3D cultures occurs spontaneously in these cultures in the course of directed differentiation to hindgut; spheroids exhibiting hindgut markers bud out of the monolayer cultures and can be easily isolated from the culture supernatants. Similar to primary tissue-derived organoids, the partially differentiated spheroids are then embedded in Matrigel® domes and incubated in cell culture medium that promotes their differentiation to the intestinal lineage and subsequent partial maturation.3
Experimental Techniques Applied to Organoid Cultures
The widespread uptake and application of intestinal organoid cultures across a variety of fields is in great part due to the multitude of experimental tools and approaches to which these cultures are amenable. Since the introduction of the basic organoid culture methodology, there have been many advances both developing and validating various techniques in the intestinal organoid model system.
Genetic Manipulation
A key functionality of the intestinal organoid system is the ability to manipulate the genetic identity of the cells that comprise the culture, especially in comparison to the challenges or infeasibility of similar manipulation performed in in vivo models. Intestinal organoid cultures can be manipulated genetically through a variety of means, including the introduction of genetic material via retro-, adeno- and lentiviral infection27–29 or electroporation,30 as well as specific site-directed mutagenesis using the CRISPR/Cas9 gene editing technique.31 Once subjected to a specific technique for genetic modification, organoid cultures can be reestablished clonally from single-cell suspensions of sorted stem cells1,2 or whole dissociated organoids, thus enabling isolation of the desired genetic variant for further propagation in 3D culture. For the mouse intestinal model, this presents a significant advantage over the time and energy required to create a specific knock-out mouse strain. For the human intestinal model, the ability to manipulate the genetic makeup of the cells in vitro presents the opportunity to exercise a level of control over the genetic composition of the model that would otherwise be impossible using traditional methods of primary cell culture or immortalized cell lines.
In addition to their compatibility with direct genetic manipulation in vitro, organoid cultures are also a highly informative way to examine pre-existing knockout models to probe the mechanisms of specific cellular processes or disease pathologies.
Biobanking
Intestinal organoid cultures are highly passageable while maintaining a remarkable level of genetic and phenotypic stability.32 However, it is sometimes advantageous to create a static library of organoid cultures through cryopreservation of the cultures. This is especially true in the context of creating cryopreserved libraries of patient-derived organoids that maintain the properties of the original culture and can be used in applications like preclinical drug efficacy screening.23 The thawed cells typically have a short (one- to two-passage(s)) recovery period during which they exhibit a decreased growth rate and after which they resume their pre-freezing growth dynamics.
Co-culture Systems
The ability to study the interaction of the epithelial cells that form the backbone of the intestinal organoid culture system with other specific cell types that can be introduced into the culture system constitutes another significant advantage of the organoid model system. The isolation of the epithelium (or epithelium with associated mesenchyme in the case of hPSC-derived organoids) from other bodily systems in the organoid culture system presents an advantage to experimental specificity, but also a limitation with respect to investigating specific interactions between this epithelium and other autologous or foreign cells. This issue is being addressed by the constant development of new co-culture systems for intestinal organoids with various other cell types. Intestinal organoids have been cultured with immune cells10,11,33 by introduction of the non-epithelial cells in the extracellular matrix supporting the organoid culture, thus recapitulating the interactions expected to take place at the basolateral side of the epithelium. A functional enteric nervous system has also been generated through the co-culture of hPSC derived organoids and neural crest cells establishing a model allowing the study of human gastrointestinal motility disorders.34 Similarly, live bacteria have been introduced into the lumen of organoid cultures via microinjection to model interactions that occur at the apical side of the epithelium in vivo.12–14 Organoids have also been validated as model systems for viral infection.15,16
Applications of Intestinal Organoids
Cell Biology
The roots of the organoid culture system lie within the developmental biology community. While this research field informed the factors necessary to develop the organoid culture model systems, intestinal organoid cultures are now feeding back valuable information about the developmental trajectory of the tissue they were designed to model.35 This is especially true for hPSC-derived intestinal organoids, which follow the overall intestinal developmental pathways during their establishment3 and maintain a fetal-like phenotype in organoid culture.36 The great utility of organoid cultures in this context lies in part in the facile accessibility to a continuum of developmental timepoints, a stark contrast to what is otherwise available with respect to human samples.
Perhaps the most obvious application of intestinal organoid cultures is as an in vitro model system for the intestinal epithelium. As these cultures both incorporate all of the epithelial cell types present in the in vivo tissue and recapitulate many if not most of the inter- and intra-epithelial cell dynamics that occur in vivo, intestinal organoid cultures constitute a valuable experimental tool for probing intestinal epithelial cell biology. As such, this in vitro model system has recently been used to investigate intestinal regeneration,37,38 the intestinal stem cell niche,39–44 intestinal inflammation,9,45–48 intestinal stem cell mutation rates49 and the function of terminally differentiated intestinal epithelial cells (eg. nutrient sensing and hormone secretion).50–52
Intestinal organoids in particular among organoid culture systems also have applications outside of tissue-specific biological processes and conditions. This is due to the active stem cell population and resultant high turnover of the intestinal epithelium compared to many other tissues. The active nature of the intestinal stem cell niche lends it great utility as a model system to investigate more general questions regarding adult stem cell biology or epithelial cell biology. Specific examples of applications that this technology has recently been applied to include investigating the shared mechanisms between adult stem cell proliferation and cancer,53 mechanisms of protein degradation,54 and epithelial polarization.55
Disease Modeling
Intestinal organoid cultures are now being used to model intestinal diseases as well as epithelial diseases in other tissues. For instance, organoids grown from patients carrying mutations in the myosin 5b gene can be used as a model for microvillus inclusion disease (MVID) which results in improper localization of proteins on the apical surface of the intestinal epithelium and enterocyte polarization.8 This model allowed for organoids to be generated from patients exhibiting MVID symptoms but not carrying mutations in the myosin 5b gene. When sequenced, these samples identified the syntaxin 3 gene as a contributor to the loss of microvilli.
Cultures can be derived from adjacent healthy and diseased (tumorigenic) tissue obtained from the same patient,23 an approach that enables evaluation of the disease state while controlling for potentially confounding factors in the healthy specific genetic background. The analysis of the genetic composition of these unique tumors allow for correlations to be made between organoid phenotype and specific mutation combinations. Multiple studies have demonstrated both the detection of mutations in the combination of APC, KRAS, SMAD4 and TP53,21 as well as used gene editing through the CRISPR/Cas9 system to model the adenoma-carcinoma sequence.31
The colon can also be exploited for modeling diseases not specific to the intestine such as Cystic Fibrosis, which usually manifests in the lung as a result of mutations in the cystic fibrosis transmembrane receptor (CFTR). Biopsies from patients carrying mutations in the CFTR gene do not respond to an organoid swelling assay, allowing for the positive identification of such mutations.56
Drug Development and Screening
One of the most promising aspects of research using organoids as a model is the possibility to screen cultures derived from individual patient tissue samples for effects of external treatments. With the quick culture times and ability to scale up samples, organoid culture has already been used in specific patient screening. For example, the previously mentioned CFTR swelling assay, designed by Beekman and colleagues,56 which provides a readout for response to current drugs or combinations of drugs which improve the transport of chloride through ion channels in patients with Cystic Fibrosis. In the first trial of the CFTR swelling assay, a drug combination able to clear much of the mucus that had accumulated in the patient's lung was identified, therefore providing a proof of principle experimental design that is currently moving towards being adopted into government screening platforms.
A key aspect that is still being optimized in organoid culture conditions is the transition to high throughput screening. Steps toward this were recently demonstrated by the development of an automated platform in 384-well format for organoid cultures established from tissue samples of colon cancer patients.24 These organoids were subjected to various drug compounds, and the assay validated for robustness and reproducibility, therefore providing a system that provides more physiological relevance than 2D cultures without sacrificing screening ability.
A reciprocal approach addresses more specific treatments for the mutation composition of patient sample libraries using a specific drug. The van Lohuizen group treated organoid cultures established using tissue samples taken from 18 patients diagnosed with colorectal cancer with an EZH2 inhibitor, GSK126.28 Although a variety of responses were observed, associations with ATRX and PAX2, as well as a correlation with BIK expression and Nutlin-3A were found. These findings demonstrate the feasibility of upstream pharmaceutical development based on patient organoid library screening.
Outlook for Intestinal Organoids
Since the recent discovery that intestinal stem cells can establish organoid structures recapitulating the mammalian intestine, the organoid model has been quickly adopted by the research community. This rapid acceptance of organoids as a research tool has been facilitated by two key aspects, the physiological relevance to the intestine and the improvements over traditional two-dimensional culture systems. For translational-based research, organoids provide the possibility of high throughput analysis of samples from individual patients bridging the gap between basic research and precision medicine. The ability to generate samples for marker or genome-based analyses, as well as drug or small molecule treatment, are possible in the same experimental design.
These cultures also provide the promise of expanding the understanding of basic stem cell biology which will be a necessary complement to the translational studies. By the very nature of the culturing system, early stem cell divisions and tissue formation will be accessible to experimental assay and manipulation via CRISPR/Cas9 gene editing.
As more organoid-based systems are established, a combining of several specific cell types into the same organoids becomes feasible. This co-culturing will allow for the identification of the specific roles of each component stem cell niche as well as the co-operative and necessary roles of the individual systems.
The applications of the organoid culture system continue to expand, and as they do so will our understanding of the complex interactions of the organs that comprise the human body, the mechanisms that have malfunctioned in specific diseases and the ability to rapidly treat patients of those diseases.
- Sato T et al. (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium Gastroenterology 141(5): 1762–72.
- Sato T et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche Nature 459(7244): 262–5.
- Spence JR et al. (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro Nature 470(7332): 105–9.
- Cong L et al. (2013) Multiplex genome engineering using CRISPR/Cas systems Science 339(6121): 819–23.
- Mali P et al. (2013) RNA-guided human genome engineering via Cas9 Science 339(6121): 823–6.
- Beekman JM. (2016) Individualized medicine using intestinal responses to CFTR potentiators and correctors Pediatr Pulmonol 51(S44): S23–34.
- Wells JM & Spence JR. (2014) How to make an intestine Development 141(4): 752–60.
- Wiegerinck CL et al. (2014) Loss of syntaxin 3 causes variant microvillus inclusion disease Gastroenterology 147(1): 65–8.
- Dotti I et al. (2016) Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis Gut. Epub ahead of print, DOI: 10.1136/gutjnl-2016-312609.
- Lindemans CA et al. (2015) Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration Nature 528(7583): 560–4.
- Nozaki K et al. (2016) Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes J Gastroenterol 51(3): 206–13.
- Leslie JL et al. (2015) Persistence and toxin production by clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function B A McCormick (Ed) Infect Immun 83(1): 138–45.
- Wilson SS et al. (2015) A small intestinal organoid model of non-invasive enteric pathogen–epithelial cell interactions Mucosal Immunol 8(2): 352–61.
- Zhang Y-G et al. (2014) Salmonella-infected crypt-derived intestinal organoid culture system for host-bacterial interactions Physiol Rep 2(9): e12147.
- Finkbeiner SR et al. (2012) Stem cell-derived human intestinal organoids as an infection model for rotaviruses MBio 3(4): e00159-12.
- Yin Y et al. (2015) Modeling rotavirus infection and antiviral therapy using primary intestinal organoids Antiviral Res 123: 120–31.
- Barker N et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5 Nature 449(7165): 1003–7.
- van de Wetering M et al. (2015) Prospective derivation of a living organoid biobank of colorectal cancer patients Cell 161(4): 933–45.
- Behjati S et al. (2014) Genome sequencing of normal cells reveals developmental lineages and mutational processes Nature 513(7518): 422–5.
- Jung P et al. (2011) Isolation and in vitro expansion of human colonic stem cells Nat Med 17(10): 1225–7.
- Drost J et al. (2015) Sequential cancer mutations in cultured human intestinal stem cells Nature 521(7550): 43–7.
- Fujii M et al. (2015) Efficient genetic engineering of human intestinal organoids using electroporation Nat Protoc 10(10): 1474–85.
- Vlantis K et al. (2016) NEMO prevents RIP kinase 1-mediated epithelial cell death and chronic intestinal inflammation by NF-κB-dependent and -independent functions Immunity 44(3): 553–67.
- Boehnke K et al. (2016) Assay establishment and validation of a high-throughputscreening platform for three-dimensional patient-derived colon cancer organoid cultures J Biomol Screen 21(9): 931–41.
- Gjorevski N et al. (2016) Designer matrices for intestinal stem cell and organoid culture Nature 539(7630): 560–4.
- Tsai Y-H et al. (2016) In vitro patterning of pluripotent stem cell-derived intestine recapitulates in vivo human development Development. Epub ahead of print, DOI: 10.1242/dev.138453.
- Andersson-Rolf A et al. (2014) A video protocol of retroviral infection in primary intestinal organoid culture J Vis Exp (90): e51765.
- Koppens MA et al. (2016) Large variety in a panel of human colon cancer organoids in response to EZH2 inhibition Oncotarget 7(43): 69816-28.
- Tao L et al. (2016) Frizzled proteins are colonic epithelial receptors for C. difficile toxin B Nature 538(7625): 350–5.
- Fujii M et al. (2016) A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis Cell Stem Cell 18(6): 827–38.
- Matano M et al. (2015) Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids Nat Med 21(3): 256–62.
- Yui S et al. (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell Nat Med 18(4): 618–23.
- Ibiza S et al. (2016) Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence Nature 535(7612): 440–3.
- Workman MJ et al. (2016) Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system Nat Med 23(1): 49-59.
- Huch M & Koo B-K. (2015) Modeling mouse and human development using organoid cultures Development 142(18): 3113–25.
- Aurora M & Spence JR. (2016) hPSC-derived lung and intestinal organoids as models of human fetal tissue Dev Biol 420(2): 230–8.
- Gregorieff A et al. (2015) Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer Nature 526(7575): 715–8.
- Oudhoff MJ et al. (2016) SETD7 controls intestinal regeneration and tumorigenesis by regulating Wnt/β-catenin and Hippo/YAP signaling Dev Cell 37(1): 47–57.
- Azzolin L et al. (2014) YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response Cell 158(1): 157–70.
- Farin HF et al. (2016) Visualization of a short-range Wnt gradient in the intestinal stem-cell niche Nature 530(7590): 340–3.
- Schuijers J et al. (2015) Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts Cell Stem Cell 16(2): 158–70.
- Schewe M et al. (2016) Secreted phospholipases A2 are intestinal stem cell niche factors with distinct roles in homeostasis, inflammation, and cancer Cell Stem Cell 19(1): 38–51.
- Igarashi M & Guarente L. (2016) mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction Cell 166(2): 436–50.
- Sasaki N et al. (2016) Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon Proc Natl Acad Sci U S A 113(37): E5399-407.
- Hibiya S et al. (2016) Long-term inflammation transforms intestinal epithelial cells of colonic organoids J Crohns Colitis. Epub ahead of print, DOI: 10.1093/ecco-jcc/jjw186.
- Zhu X et al. (2015) Cytosolic HMGB1 controls the cellular autophagy/apoptosis checkpoint during inflammation J Clin Invest 125(3): 1098–110.
- López-Posadas R et al. (2016) Rho-A prenylation and signaling link epithelial homeostasis to intestinal inflammation J Clin Invest 126(2): 611–26.
- Weeber F et al. (2015) Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases Proc Natl Acad Sci U S A 112(43): 13308–11.
- Blokzijl F et al. (2016) Tissue-specific mutation accumulation in human adult stem cells during life Nature 538(7624): 260–4.
- Zietek T et al. (2015) Intestinal organoids for assessing nutrient transport, sensing and incretin secretion Sci Rep 5: 16831.
- Petersen N et al. (2015) Targeting development of incretin-producing cells increases insulin secretion J Clin Invest 125(1): 379–85.
- In JG et al. (2016) Human mini-guts: new insights into intestinal physiology and host-pathogen interactions Nat Rev Gastroenterol Hepatol 13(11): 633–42.
- Man SM et al. (2015) Critical role for the DNA sensor AIM2 in stem cell proliferation and cancer Cell 162(1): 45–58.
- Sun S et al. (2015) IRE1α is an endogenous substrate of endoplasmic-reticulum-associated degradation Nat Cell Biol 17(12): 1546–55.
- Nakajo A et al. (2016) EHBP1L1 coordinates Rab8 and Bin1 to regulate apical-directed transport in polarized epithelial cells J Cell Biol 212(3): 297–306.
- Dekkers JF et al. (2013) A functional CFTR assay using primary cystic fibrosis intestinal organoids Nat Med 19(7): 939–45.
Researcher Profile: Tamara Zietek, PhD
Principal Investigator at the Technical University of Munich
Researcher Profile: Jason Spence, PhD
Associate Professor at the University of Michigan
Caroline Lindemans, MD, PhD
Clinician Scientist, University Medical Center Utrecht, Netherlands
Patient-Derived Organoids for Drug Screening and Development
Watch the webinar presented by Dr. Sylvia Boj
Podcast: Dr. Caroline Lindemans
Dr. Caroline Lindemans talks about her research on the role of innate immune cells in intestinal stem cell maintenance.
Modeling Human Gastrointestinal Development and Disease Using Pluripotent Stem Cells
Watch the webinar presented by Dr. James Wells
Organoids Podcast: Jason Spence
Jason Spence shares his insights on the derivation of gastrointestinal organoids from hPSCs
Organoids Podcast: Tamara Zietek
Tamara Zietek discusses the use of organoids to study nutrient sensing and transport
Key Applications of Intestinal Organoids
Crypt-Derived Intestinal Organoids
Ootani A et al. (2009) Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15(6): 701–6.
Sato T et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244): 262–5.
Sato T et al. (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141(5): 1762–72.
PSC-Derived Intestinal Organoids
Intestinal Cell Biology
Dotti I et al. (2016) Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut. Epub ahead of print, DOI: 10.1136/gutjnl-2016-312609.
Igarashi M & Guarente L. (2016) mTORC1 and SIRT1 cooperate to foster expansion of gut adult stem cells during calorie restriction. Cell 166(2): 436–50.
Oudhoff MJ et al. (2016) SETD7 controls intestinal regeneration and tumorigenesis by regulating Wnt/β-catenin and Hippo/YAP signaling. Dev Cell 37(1): 47–57.
Sasaki N et al. (2016) Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc Natl Acad Sci U S A 113(37): E5399-407.
Schewe M et al. (2016) Secreted phospholipases A2 are intestinal stem cell niche factors with distinct roles in homeostasis, inflammation, and cancer. Cell Stem Cell 19(1): 38–51.
Schwank G et al. (2013) Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13(6): 653–8.
Workman MJ et al. (2016) Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. Epub ahead of print, DOI: 10.1038/nm.4233.
Yui S et al. (2012) Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med 18(4): 618–23.
Zietek T et al. (2015) Intestinal organoids for assessing nutrient transport, sensing and incretin secretion. Sci Rep 5: 16831.
Epithelial Cell Biology
Horvay K et al. (2015) Snai1 regulates cell lineage allocation and stem cell maintenance in the mouse intestinal epithelium. EMBO J 34(10): 1319–35.
Kikuchi I et al. (2016) Dephosphorylated parafibromin is a transcriptional coactivator of the Wnt/Hedgehog/Notch pathways. Nat Commun 7: 12887.
Nakajo A et al. (2016) EHBP1L1 coordinates Rab8 and Bin1 to regulate apical-directed transport in polarized epithelial cells. J Cell Biol 212(3): 297–306.
Cancer Research
Fernández-Majada V et al. (2016) The tumour suppressor CYLD regulates the p53 DNA damage response. Nat Commun 7: 12508.
Fujii M et al. (2016) A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18(6): 827–38.
Koo B-K et al. (2015) Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc Natl Acad Sci U S A 112(24): 7548–50.
Liu W et al. (2016) Olfactomedin 4 deletion induces colon adenocarcinoma in Apc(Min/+. Oncogene 35(40): 5237–47.
van der Heijden M et al. (2016) Bcl-2 is a critical mediator of intestinal transformation. Nat Commun 7: 10916.
Yan HHN et al. (2016) RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut. Epub ahead of print, DOI: 10.1136/gutjnl-2016-311849.
Yum MK et al. (2016) AIMP2 controls intestinal stem cell compartments and tumorigenesis by modulating Wnt/β-catenin signaling. Cancer Res 76(15): 4559–68.
Cystic Fibrosis Research
Dekkers JF et al. (2016) Optimal correction of distinct CFTR folding mutants in rectal cystic fibrosis organoids. Eur Respir J 48(2): 451–8.
Vijftigschild LAW et al. (2016) β2-Adrenergic receptor agonists activate CFTR in intestinal organoids and subjects with cystic fibrosis. Eur Respir J 48(3): 768–79.
Intestinal Immunity and Microbiomics
Gerbe F et al. (2016) Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529(7585): 226–30.
Hohwieler M et al. (2016) “Miniguts” from plucked human hair meet Crohn’s disease. Z Gastroenterol 54(8): 748–59.
Ibiza S et al. (2016) Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535(7612): 440–3.
Lindemans CA et al. (2015) Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528(7583): 560–4.
Oancea I et al. (2016) Colonic microbiota can promote rapid local improvement of murine colitis by thioguanine independently of T lymphocytes and host metabolism. Gut 66(1): 59–69.
Peuker K et al. (2016) Epithelial calcineurin controls microbiota-dependent intestinal tumor development. Nat Med 22(5): 506–15.
Wang Y et al. (2016) An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunol. Epub ahead of print, DOI: 10.1038/mi.2016.57.
Wilson SS et al. (2015) A small intestinal organoid model of non-invasive enteric pathogen–epithelial cell interactions. Mucosal Immunol 8(2): 352–61.
Bacterial and Viral Pathogenesis
In JG et al. (2016) Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nat Rev Gastroenterol Hepatol 13(11): 633–42.
Tao L et al. (2016) Frizzled proteins are colonic epithelial receptors for C. difficile toxin B. Nature 538(7625): 350–5.
Compound Screening
Zomer-van Ommen DD et al. (2016) Functional characterization of cholera toxin inhibitors using human intestinal organoids. J Med Chem 59(14): 6968–72.