Media and Supplements for In Vitro Hematotoxicity Testing
- Document # 27029
- Version 1.0.4
- May 2023
Background
Many drugs have adverse effects on the ability of the bone marrow (BM) to sustain blood cell formation. These effects can be life-threatening for patients, while limiting the overall effectiveness and clinical utility of a drug. For this reason quantitative in vitro methods for measuring the hematotoxicity of drug candidates on normal hematopoietic stem and progenitor cells (HSPCs) are essential in the drug development process.
HSPCs are readily accessible from BM and blood. Traditionally, in vitro colony-forming unit (CFU) assays using BM or umbilical cord blood (CB) have been the gold standard for measuring hematotoxicity and have shown their utility in predicting the toxicity of drugs on blood cells in vivo.1,2 The CFU assay is most useful at late stages of drug development when the pool of drug candidates has been reduced to a size conducive to this informative but resource intensive assay. New liquid culture-based assays in a multi-well format are often more suitable for testing larger numbers of compounds at earlier stages of development.
Media and Supplements for In Vitro Hematoxicity Testing
STEMCELL has developed three HemaTox™ kits for testing the effect of drugs on the growth and lineage-specific differentiation of human HSPCs in 96-well culture plates.
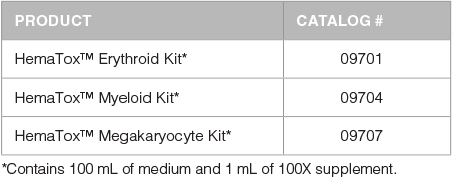
Each kit is formulated to test the effect of drugs on the outgrowth of specific progenitor cell lineages. The HemaTox™ Erythroid Kit (Catalog #09701), HemaTox™ Myeloid Kit (Catalog #09704) and HemaTox™ Megakaryocyte Kit (Catalog #09707) are designed to test erythroid-, myeloid- and megakaryocyte-specific toxicity, respectively, and can potentially be used to predict the ability of drugs to cause anemia, neutropenia or thrombocytopenia in vivo. Each kit contains a specialized serum-free culture medium and 100X supplement which have been optimized to promote the proliferation and differentiation of human CD34+ hematopoietic progenitor cells into each lineage. Each kit contains sufficient medium and supplement to plate cells in five 96-well plates, allowing users to assay ~150 - 250 compounds per kit, depending on the number of replicates and controls.
Products
Advantages
- Produces IC50 values similar to those determined by an appropriate CFU assay, the current standard in vitro assay for measuring hematotoxicity.
- Culture conditions result in lineage-specific differentiation of CD34+ HSPCs into erythroid, myeloid or megakaryocyte progenitor cells.
- Test compounds can be added at the start of culture to examine effects on progenitors before differentiation, or later during culture to study effects on more differentiated cells. Different readout methods may be used, e.g., flow cytometry or plate reader.
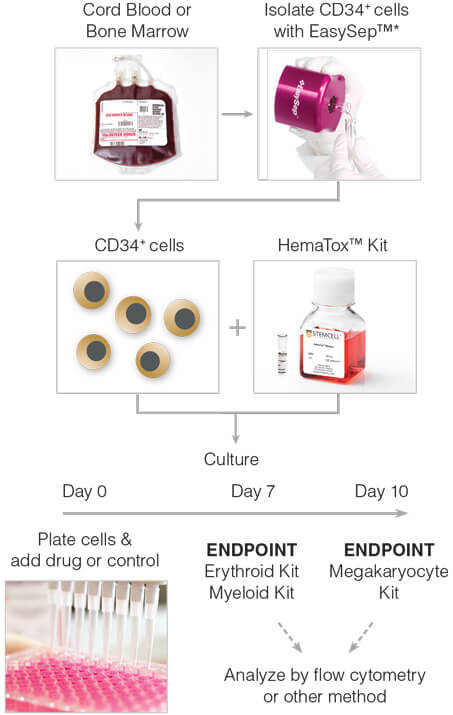
Figure 1. General HemaTox™ Kit Procedure
*The cell isolation step may be omitted if pre-enriched CD34+ cells are used.
General Method for In Vitro Liquid Culture-Based Hematotoxicity Assay
The following protocol is recommended for the expansion and lineage-specific differentiation of human BM or CB CD34+ cells with HemaTox™ kits, in order to determine the toxicity of compounds on erythroid, myeloid or megakaryocytic cells. For detailed protocols please consult the product information sheet (PIS) for each kit, available at www.stemcell.com.
- Prepare enough complete medium (HemaTox™ Medium + 100X Supplement) for a single experiment.
- Dilute test compound in complete medium.
- Prepare solvent controls in complete medium.
- Isolate CD34+ cells* from human BM or CB. Freshly isolated CD34+ cells can be used immediately or aliquots may be cryopreserved for later use. CD34+ cells may be isolated from:
- BM mononuclear cells using the EasySep™ Human CD34 Positive Selection Kit (Catalog #18056).
- Whole CB using the EasySep™ Human Cord Blood CD34 Positive Selection Kit II (Catalog #17896).
*This step may be omitted if pre-enriched CD34+ cells are used.
- Plate cells in complete medium in a 96-well flat bottom plate.
- Add test compounds and controls to wells.
- Place each 96-well plate in a humidity chamber (e.g. a square dish (Catalog #38039) surrounded by four 35 mm dishes (Catalog #27100) filled with sterile water).
- Incubate at 37°C at 5% CO2 for:
- 7 d ays - HemaTox™ Erythroid and Myeloid Kits
- 10 days - HemaTox™ Megakaryocyte Kit
- Analyze by flow cytometry or an alternative readout method.
Analysis
Users can choose an appropriate readout method to measure the effects of test compounds on HSPC proliferation and lineage-specific differentiation. The recommended method involves staining cultured cells for lineage-specific cell surface markers and counting cells that express these markers using a flow cytometer with absolute cell counting capability. This enables users to quantify the response and obtain estimates for the 50% and 90% inhibitory concentrations (IC50 and IC90, respectively) of each test compound in the assay. Alternative readout methods (e.g. automated cell counting, imaging cytometry or plate readerbased methods) can also be used, but may require further optimization.
Table 1. Antibody Staining Panel for Immunophenotyping by Flow Cytometry
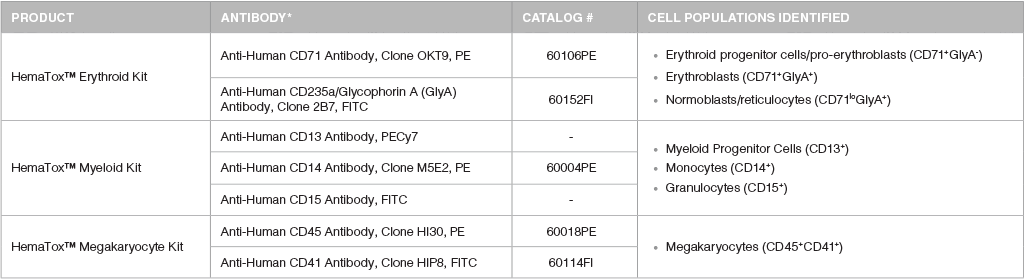
*Recommended flurochromes are shown; however, users should select the fluorochrome that is appropriate for their instrument and analysis.
Analysis of Cells Produced in Culture
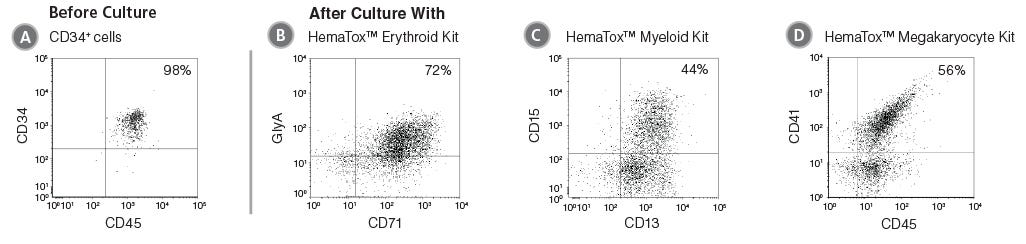
Figure 2. Flow Cytometry Plots Showing Cells Produced After Culture of CD34+ HSPCs with HemaTox™ Erythroid, Myeloid and Megakaryocyte Kits
(A) Human CB CD34+ cells were cultured with (B) HemaTox™ Erythroid, (C) Myeloid and (D) Megakaryocyte Kits using the protocol above. After the appropriate culture period, cells were harvested and stained for cell surface proteins expressed on erythroid (CD71 and GlyA), myeloid (CD13 and CD15) or megakaryocytic (CD45 and CD41) cells, respectively.
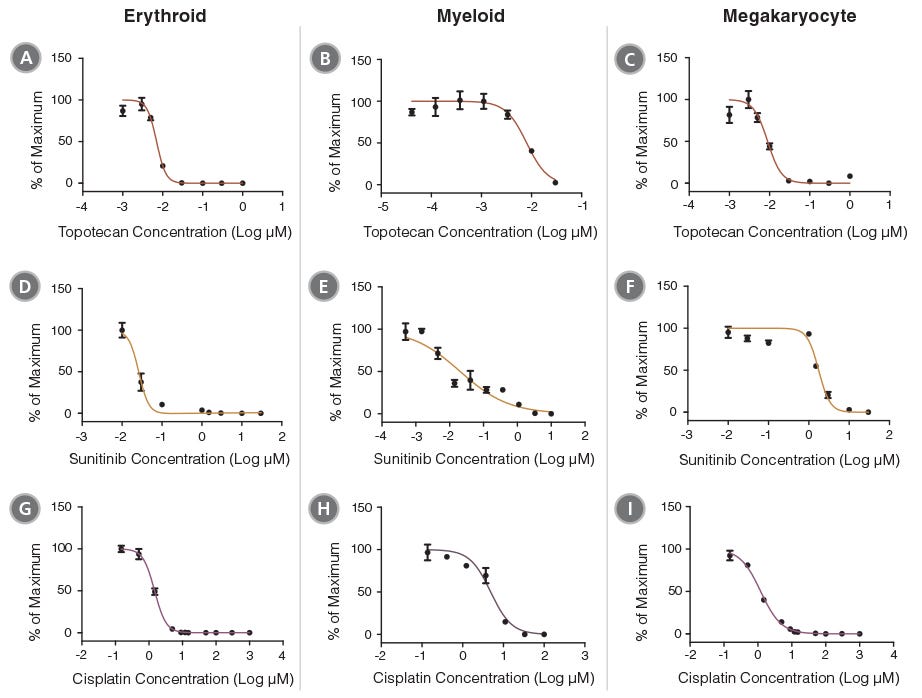
Figure 3. Concentration- Dependent Inhibition of Hematopoietic Progenitor Cell Outgrowth with HemaTox™ Erythroid, Myeloid and Megakaryocyte Kits
Dose-response curves were produced for a selection of 6 drugs using the HemaTox™ Erythroid, Myeloid and Megakaryocyte Kits. These curves can be used to calculate IC50 and IC90 values for each drug. Cell numbers at each drug concentration were normalized to the percentages (%) of maximum cell growth. Two topoisomerase inhibitors (Topotecan [A,B,C] and Irinotecan [not shown]), two tyrosine kinase inhibitors (Sunitinib [D,E,F] and Imatinib [not shown]) and two anti-metabolites (Cisplatin [G,H,I] and 5-Fluorouracil [5-FU, not shown]) were evaluated using human BM CD34+ cells according to the protocol for each kit (see PIS or protocol on page 2). Each data point represents the average of 3 - 6 replicate cultures (vertical bars = standard error of the mean).
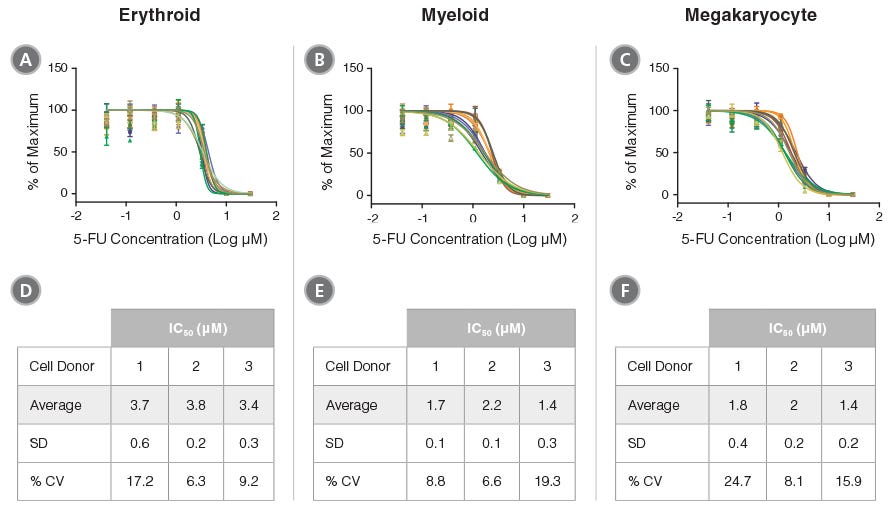
Figure 4. Reproducibility of HemaTox™ Kit Results Between Experiments and Using Different CD34+ Cell Preparations
Dose-response curves were generated from titrations of 5-FU added to human CB CD34+ cells isolated from 3 donors and cultured with the HemaTox™ (A,D) Erythroid, (B,E) Myeloid and (C,F) Megakaryocyte Kits. Three to five separate experiments were performed with cells from each donor. In each assay similar IC50 values were obtained with cells from different donors and in different experiments with cells from the same donor. Shown are values normalized to the percentages (%) of maximum cell growth without the drug. Despite differences in absolute cell counts, curves are reproducible when normalized within each experiment. (D,E,F) Tables showing IC50 values generated for 5-FU in culture with each kit including standard deviation (SD) and the coefficient of variation (% CV) across experiments.
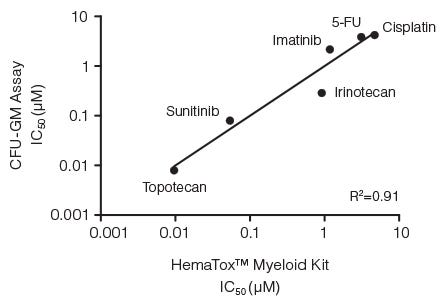
Figure 5. Correlation Between IC50 Values for Six Drugs Measured Using the CFU-GM Assay and the 96-Well Plate Liquid Culture-Based HemaTox™ Myeloid Kit
Human BM CD34+ cells were cultured in colony-forming unit - granulocyte/ macrophage (CFU-GM) assays with MethoCult™ medium and in liquid culture with the HemaTox™ Myeloid Kit. IC50 values generated using each assay were plotted on the X and Y axes and shown to be highly correlated with a coefficient of determination (R2) of 0.91.
Lineage-Specific Differences in Hematotoxicity
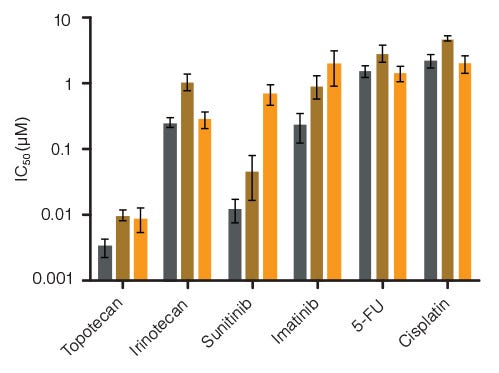
Figure 6. Lineage-Specific Differences in Hematotoxicity Identified with HemaTox™ Erythroid, Myeloid and Megakaryocyte Kits
Results show average IC50 values for each drug tested on human BM CD34+ cells using the HemaTox™ Erythroid (grey), Myeloid (gold) and Megakaryocyte (orange) Kits. Most drugs show similar toxicity for each lineage but some, such as Sunitinib, are ~100-fold more toxic for erythroid than for megakaryocyte progenitor differentiation with intermediate toxicity for myeloid progenitor differentiation. Vertical lines indicate standard error of the mean (SEM) (n = 4 - 8). See Table 2 for specific IC50 values determined using the CFU-GM assay vs. HemaTox™ kits for Sunitinib.
For available fresh and cryopreserved peripheral blood, cord blood and bone marrow products in your region, visit www.stemcell.com/primarycells.
Table 2. Comparison of IC50 Values for Sunitinib as Determined Using CFU Assay and HemaTox™ Kits
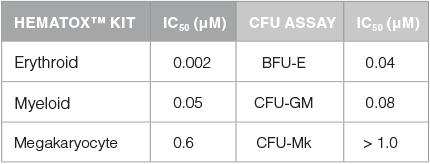
IC50 values were generated in CFU assays for lineage-specific progenitor cells by plating human BM CD34+ cells in MethoCult™ media that supports the growth of erythroid progenitor cells (BFU-E: burst-forming unit - erythroid) or myeloid progenitor cells (CFU-GM: colony-forming unit - granulocyte/macrophage), or in MegaCult™-C medium that supports the growth of megakaryocyte progenitor cells (CFU-Mk: colony-forming unit - megkaryocyte). Cells were also cultured with the HemaTox™ Erythroid, Myeloid and Megakaryocyte Kits. IC50 values were generated for all 6 assays. These results show that the erythroid lineage is ~100- fold more sensitive to Sunitinib than the megakaryocyte lineage. Additionally, the IC50 measured with the HemaTox™ Erythroid Kit was ~10-fold lower than the IC50 measured in the CFU assay for erythroid progenitor cells, BFU-E. This indicates that for certain drugs the HemaTox™ kits may identify toxicities that are less apparent when using classical CFU assays.
Accessory Products
References
- Pessina A et al. (2003) Toxicol Sci 75:355-367.
- Pessina A et al. (2009) Toxicol In Vitro 23(1):194-200.
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration

