Evaluating the Performance of Cell Separation Methods
There are many parameters to consider when evaluating the performance of your cell separation procedure. These parameters can be helpful to compare when deciding which cell isolation method to use. Key performance measures such as purity and recovery are important to consider and assess. Other critical parameters may vary depending on the intended downstream application for the isolated cells. Here we outline some of the parameters that you may want to consider when choosing a cell separation method.
Purity
Purity refers to the proportion of desired cells in the final isolated cell fraction, and is generally expressed as a percentage of total live cells. Purity is an important criterion for evaluating the success of cell separation because it indicates whether the final isolated cell population sufficiently represents the characteristics of that particular cell type without the interfering effects of other cell types.
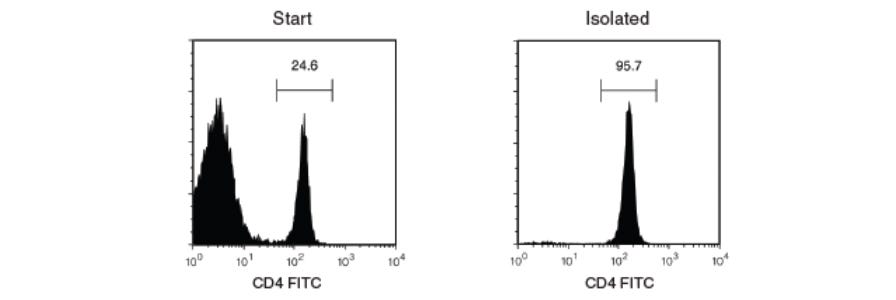
Purity is most commonly measured using flow cytometry, in which cells are labeled with fluorescent markers and analyzed using a flow cytometer. See these considerations for FACS gating when analyzing your sample.
When preparing cells for analysis by flow cytometry, staining antibodies should be chosen carefully, taking into consideration the specific cell type(s) involved and cell separation method used (negative or positive selection). Cells separated by immunomagnetic negative selection can be stained using antibodies against the primary cell surface marker epitope(s). Antibodies used to label cells for positive selection may partially or fully block epitopes on the primary cell surface marker, in which case staining antibodies targeting alternative epitopes on the target cells should be used for purity assessment. For example, if positive selection for CD3+ cells fully or partially blocks the epitope of other CD3 antibody clones, then CD4 and CD8 antibodies can instead be used for flow cytometric analysis of your isolated T cell populations. Alternatively, fluorochrome-conjugated secondary antibodies specific to the primary labeling antibodies can be used to stain isolated cells. If you’re using our cell separation products, refer to the corresponding product information sheets for recommended antibody clones that you can use to analyze your isolated cells.
Recovery
Recovery refers to the proportion of desired cells isolated to the available desired cells in the starting sample. Recovery answers the question: out of all the desired cells you can possibly obtain from your sample, how many are you actually able to isolate? Conversely, how many of your desired cells have you lost through your cell separation method? Recovery is ideally reported as a percentage of total available desired cells in the starting sample.
Although not always the case, purity and recovery tend to be inversely correlated. Researchers need to balance their requirement for highly purified cells with the number of target cells they require for their experiments. Learn how to assess recovery from cell isolation procedures >
Yield
Yield refers to the total number of recovered target cells. This can be reported as the number of isolated cells per amount of starting sample, or as the total number of desired cells in the final isolated fraction. The higher the recovery, the higher the yield. Recovery and yield can be affected by multiple factors, including the quality of the sample preparation, type of sample, starting frequency of your desired cells, heterogeneity of the start sample, and the quality of your cell isolation kit.
Viability
Viability can be expressed as the percentage of live cells out of total cells in the isolated sample. Viability is especially important when researchers need live cells for downstream cell culture and other applications. You may also want to minimize the number of dead cells in your samples because dead cells can release factors that promote cell death or stress responses from neighbouring cells.
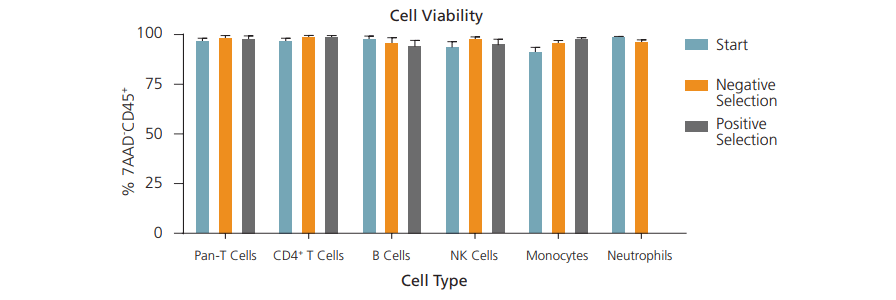
A common way to measure cell viability is to assess cell membrane integrity, as unhealthy cells have compromised cell membranes. This can be done using viability dyes, including 7-AAD and Trypan Blue, which can permeate unhealthy cells.
Function
Function of the cells you isolate should be preserved throughout the cell separation process. This ensures that your downstream in vitro or in vivo assays accurately represent the physiological function of your cell type of interest. There are many ways to evaluate the functionality of cells—the right method is dependent on your cell type of interest and specific research questions. For example, if you want to isolate functional T cells, you may want to assess the expression of cytokines in the presence and absence of stimulation to ensure the T cells respond appropriately.
Reproducibility
Reproducibility is always important in scientific research. Variability can reduce your ability to detect statistical significance and draw meaningful insights from your results. There are a number of factors that can cause variability, including protocol times, sample-to-sample variability, amount of sample manipulation, and the user performing the procedure. The quality of the cell isolation reagent you're using can also impact reproducibility.
Labeling
Labeling your cells with antibodies or ligands is a critical step for both negative and positive selection cell separation methods. With proper labeling, the antibodies or ligands and magnetic particles used should enable efficient cell isolation without interfering with downstream applications. Under-labeling of your sample can result in reduced recovery of your target cells (in positive selection) or reduced purity of your desired cells (in negative selection). Over-labeling can also be problematic due to the risk of non-specific binding of labeling reagents to non-target cells.
There are a number of steps you can take to prevent under- or over-labeling (including non-specific binding) of cells in your sample. Most manufacturers will provide detailed protocols for you to follow when using their cell separation products, which should have been carefully optimized by their product development scientists to ensure reagents are properly titrated. In addition to carefully following the manufacturer’s protocols, another way you can minimize unwanted interactions is by using an FcR blocker to prevent the Fc region of antibodies from non-specifically binding to cells.Speed
Speed refers to the amount of time it takes to complete the cell separation procedure and indirectly affects the considerations described above. Faster cell separation protocols are desirable if you need to increase your throughput or simply want to accomplish more with your time in the lab. Some of the fastest cell separation kits can isolate highly purified cells in as little as 8 minutes.
A More Efficient Way to Isolate Cells
Scientists who work efficiently accomplish more in less time and with less effort. We’ve developed a smarter way to isolate cells that takes less time and effort without compromising reliability, purity, or recovery.
Throughput
Throughput refers to the rate at which cell isolation can be completed in terms of sample volume, number of cells, or number of samples. How many cells can you isolate in one cell separation experiment? How many samples can you process simultaneously? If you're working with large sample volumes or multiple samples at a time, you will want to consider which cell isolation technology can support your desired throughput.
Ease-of-Use
Ease-of-use contributes to the reliability and reproducibility of a cell separation method, especially for new users. In one example, volunteers on an expedition to the Bolivian Andes were able to isolate cells to study the effect of altitude on the immune system, despite having no experience in cell separation and limited access to lab equipment. Simple cell isolation protocols were key in reducing user errors and variability.
Sample and Cell Types
Sample and cell types can determine which cell separation technologies are available to you. For example, there is a commercially available immunomagnetic cell isolation kit for the isolation of ILCs from mouse lungs, but there may not be one that's optimized for the isolation of Th2 cells from adipose tissue. Moreover, the sample volume you're working with may also limit the cell separation technologies available for your application.
In some cases, you may want to isolate multiple cell types from a single sample. Some cell isolation methods and technologies will allow you to perform sequential cell separation.Cost
Cost is often front-and-center when making decisions, especially if your research funding is limited. When evaluating the cost of any cell separation method, remember to include all costs, including reagents (e.g. antibodies, magnetic particles, and density gradient media), disposables (e.g. tubes, tubing, and columns), and time and service (e.g. flow cytometry core facility bookings). Cost savings should not compromise performance.
Automation
Automation can reduce variability, limit the amount of hands-on time required, and allow you to do more with your time in the lab. Automated cell separation instruments, such as RoboSep™, also reduce the risk of exposure to dangerous pathogens when working with potentially infectious samples.
RoboSep™: Automated Cell Separation
Fully automated magnetic cell separation instruments that can isolate cells from up to 4 or 16 samples simultaneously.