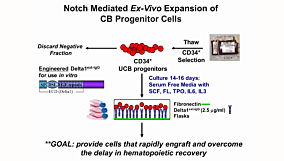
Qualification of Ancillary/Raw Materials for Clinical Use
This presentation is a step-by-step guide to qualify ancillary materials for clinical use. It will focus on the path to qualification, region-specific regulations and guidelines, common industry issues and case studies.
Publish Date:
June 20, 2018
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
By submitting this form, you are providing your consent to STEMCELL Technologies Canada Inc. and its subsidiaries and affiliates (“STEMCELL”) to collect and use your information, and send you newsletters and emails in accordance with our privacy policy. Please contact us with any questions that you may have. You can unsubscribe or change your email preferences at any time.