Human Primary Cells
What Are Primary Cells?
Human primary cells are cells isolated directly from human tissues, including blood and bone marrow, using enzymatic or mechanical methods. As primary cells are derived directly from living tissue, using these cells in your cell-based assay produces more reliable and relevant results—as they represent the in vivo biology of cells more closely. Increasingly, human primary cells are being recognized for their importance in the study of biological processes, disease progression, and drug development, and for their use in applications such as in vitro cell-based assays, xenograft creation, and humanized mouse models.
Read on to learn more about the benefits of incorporating primary cells into your research and how working with a dependable cell supplier can help streamline your cell-based assays.
Why Use Human Primary Cells?
Immortalized cell lines have long been used in in vitro cell-based assays. However, scientists acknowledge that biological changes resulting from the continuous passage of cell lines may limit their physiological relevance in studies. Human primary cells retain key aspects of their tissue of origin and reflect donor variability, including human leukocyte antigen (HLA) type and cytomegalovirus (CMV) status, more accurately than cell lines. Using human primary cells increases the physiological relevance of cell culture systems, enabling you to generate meaningful data that is more predictive of in vivo outcomes. This approach reduces the need for extensive in vivo validation and helps to facilitate the translation of basic research into preclinical or clinical applications.
Cell Lines vs. Primary Cells
Learn more about the advantages of using primary cells over immortalized cell lines in your research.
Our Human Primary Cell Products
STEMCELL Technologies has a variety of high-quality, ethically sourced primary cell products to meet your research needs. Choose ready-to-use fresh or cryopreserved cells, including mononuclear cells (MNCs), purified immune cells, or stem cells isolated from blood or bone marrow, and begin experiments when you are ready.*
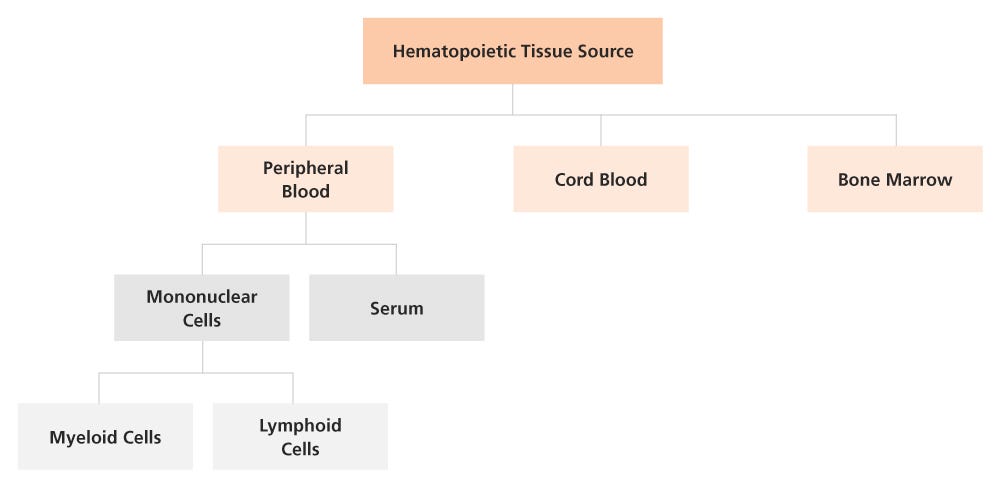
Figure 1. Sources of Human Primary Cell Offerings for Your Research
STEMCELL offers a range of fresh or frozen human primary cell products from peripheral blood, cord blood, and bone marrow to streamline your cell-based assays.
Here is an overview of our human primary cell product categories:
Peripheral Blood Products
Peripheral blood (PB)-derived normal and diseased primary cell products provide a useful tool for studying various aspects of immunology, infectious disease, hematological malignancies, vaccine development, and cell therapy and are compatible with high-throughput screening techniques.
Learn More >Umbilical Cord Blood Products
Umbilical cord blood (CB)-derived cells are an advantageous choice for developing screening assays for drug discovery, regenerative cell therapy, and immune modulation, due to their enhanced capacity for progenitor cell proliferation, multilineage differentiation, and self-renewal in vitro.
Learn More >Mobilized Peripheral Blood Products
Mobilizing PB with granulocyte colony-stimulating factor (G-CSF), plerixafor (Mozobil®), or a combination of both G-CSF and plerixafor induces hematopoietic stem and progenitor cells (HSPCs) to migrate out of the bone marrow and into the blood. Using large numbers of mobilized PB-sourced HSPCs from a single collection offers an advantageous choice to ensure consistency across multiple experiments or in large-scale studies.
Learn More >Bone Marrow Products
Human bone marrow (BM)-derived HSPCs and stromal or mesenchymal cells (MSCs) offer an attractive platform to develop drug screening assays.
Learn More >Have a question? See Frequently Asked Questions on Primary Cells for answers, or contact us directly.
Partner with the Right Primary Cell Supplier
The success of your primary cell-based research depends on partnering with the right cell supplier. Having a dependable source of quality human primary cells that consistently meet your requirements can reduce experimental uncertainty and increase confidence in your results.
At STEMCELL Technologies, our sales and support teams are committed to helping you find the right human primary cells that are ethically sourced and approved for use in your application. Read on to learn how we can work closely with you to ensure that your needs are met.
Why Use Human Primary Cells from STEMCELL Technologies?
- Choose cells that are more physiologically representative of cells in vivo.
- Access donor samples collected using regulatory authority-approved consent forms and protocols.
- Request custom products for non-standard cell types or collections with specific requirements.
- Reserve large numbers of cryopreserved cells and start experiments on your schedule with cells you’ve already tested.
- Reduce time spent collecting and culturing primary cells.
A Reliable Supply of Human Primary Cells
A reliable source of human primary cells ensures continuity in your research and allows you to start your experiments according to your schedule, without being restricted by the availability of tissue. Avoid many of the challenges often associated with obtaining human biological material by using fresh or cryopreserved cells that are ready to use upon receipt. Our Quality Assurance, Quality Control, and Regulatory Affairs departments are ready to assist you with any necessary documentation to meet specific institutional requirements.
Quality, Ethically Sourced Human Primary Cells
All of our human primary cell products are ethically sourced using Informed Consent Forms (ICFs) and protocols approved by either an Institutional Review Board, the Food and Drug Administration (FDA), the U.S. Department of Health and Human Services, and/or an equivalent regulatory authority. Donations are collected in the United States in compliance with applicable federal, state, and local laws, regulations, and guidance. Donors are prescreened for general health and viral status, including HIV-1, HIV-2, hepatitis B, and hepatitis C. Additional screening or analysis is available upon request. Learn more about our Donor Viral Screening Policy**.
Most purified cells are isolated using column-free cell isolation technology and cryopreserved in defined, serum-free media. State-of-the-art equipment, including automated cryogenic storage systems and cryogenic sample carriers, ensure cold chain-of-custody management and high sample integrity. Cells are shipped with a Certificate of Analysis indicating guaranteed quality control testing results, including cell count, viability, and purity. STEMCELL's Quality Management System is certified to ISO 13485:2016 Medical Devices and ISO 9001:2015.
Request an Offer for Human Primary Cells
Fill out this form to request information about introductory offers to try STEMCELL’s human primary cell products.
Request Offer >Frequently Asked Questions on Primary Cells
Find answers to frequently asked questions on our primary cell products.
Learn More >Donor Specifications and Characterization Services
Choose human primary cells based on donor specifications that include sex, age range, BMI range, ethnicity, blood type, smoker status, CMV, HLA, and more. Get the cells you need by reserving multiple lots for testing and also save time by letting us characterize your cells with services including high-resolution HLA typing (Class I and II), CMV status, EBV status, vaccination status, genotyping, phenotyping, and FACS analysis.
Flexible Delivery and Shipping Options
Flexible delivery options allow you to start experiments according to your schedule. Arrange for collection and delivery of fresh cells* at your convenience with our Customer Service representatives (orders@stemcell.com). Early morning delivery is available for customers based in the United States, and same-day delivery is available in select regions. Please consult your sales representative or contact us for delivery options available in your region.
Cryopreserved cells are shipped using dry ice, but liquid nitrogen dry vapor shippers are available upon request. Fresh immune cell subsets* and leukopaks are shipped at 2 - 8°C, and fresh whole blood products* are shipped at room temperature using standard boxes and delivered within 24 hours of collection. Alternatively, certified temperature boxes are available upon request. Read more about our Fresh Order Fulfillment Policy***.
Flexible Policy on Orders and Holds
Reduce the lead time for upcoming studies that may require specific donors or large quantities of cells by working closely with your sales representative. We can start the collection and manufacturing process in advance of large projects, ensuring that your cells are ready when you are.
When working with human biological material, variability between donors or sample collections is expected. Save time and effort associated with pre-screening cells from different lots before each experiment by reserving entire lots of cryopreserved cells while you test them in your assays. This ensures that your preferred cells are ready for your next experiment, without the need to prescreen again. Contact your sales representative or our primary cells team to learn more.
Regulatory Support
Learn how STEMCELL can support your regulatory and compliance needs when using human primary cells in your research.
Learn More >Custom Products
Get custom primary cell products—including unique peripheral blood isolations, collections using alternative anticoagulants, and donor-matched collections, additional testing, and more.
Learn More >For more information on our services related to STEMCELL primary cell products, please see our terms and conditions. You can also contact our primary cells team or your sales representative to discuss your specific application and regulatory or product requirements.
Workflow Solutions
A standardized cell culture system not only includes quality cells, but also quality media, supplements, reagents, and protocols. Unlike other primary cell suppliers, we have over 20 years of expertise in the development of cell isolation and cell culture reagents to support every stage of your research workflow. Our innovative technologies are used to isolate, culture, and cryopreserve your primary cells, enabling you to start your research with confidence. Explore related and compatible products for each stage of your drug discovery, hematopoietic, immunology, immuno-oncology, cell therapy, or other research.
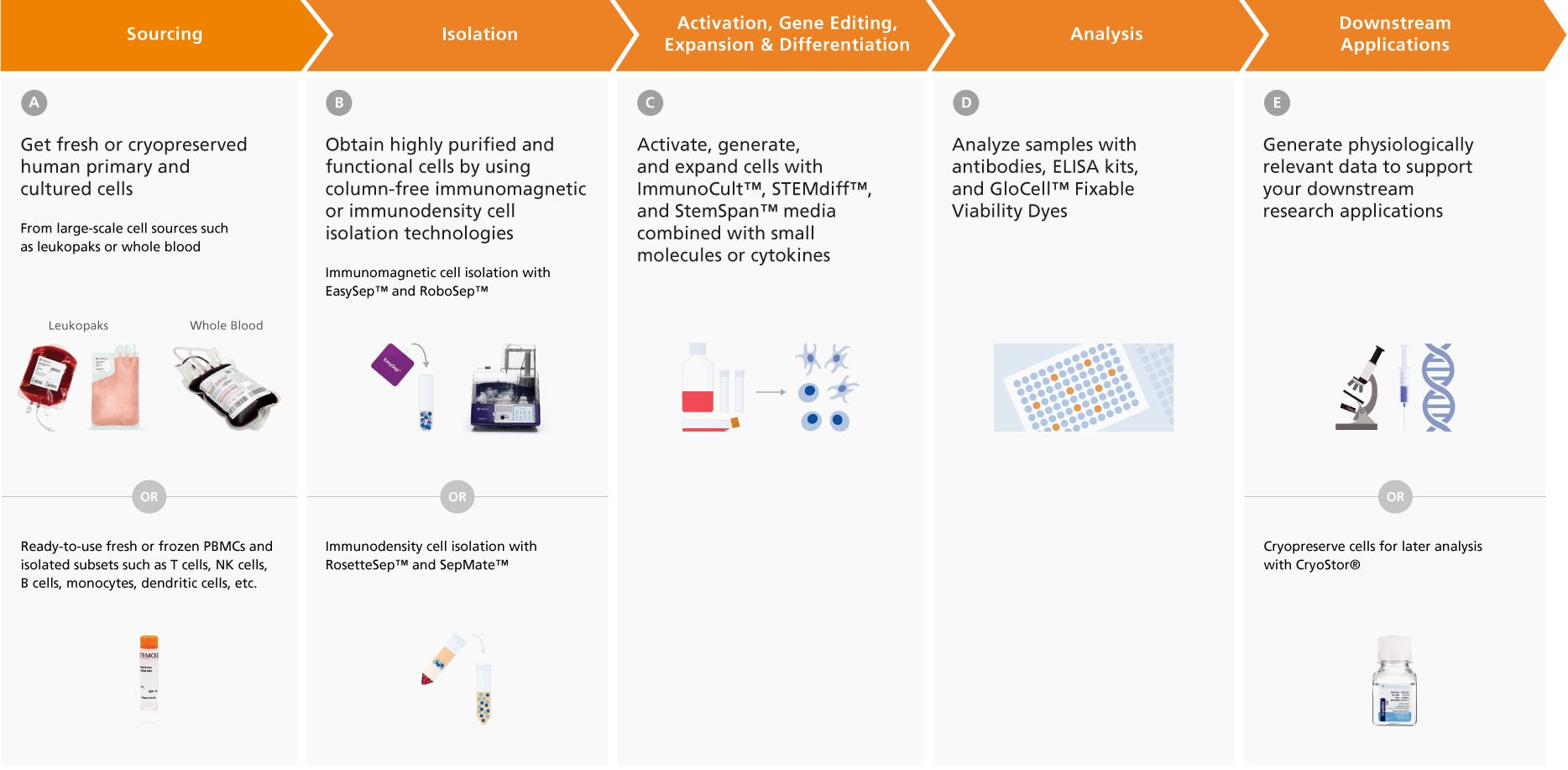
Figure 2. Integrated Workflow Solutions for Your Research
From primary human cells to cell isolation kits, culture media, supplements, antibodies, and more, STEMCELL Technologies provides the tools you need for every step of your research.
Primary Cell-Based Assay Services
Are you looking for expertise beyond your in-house drug discovery capabilities? STEMCELL's Contract Assay Services (CAS) is a contract research organization (CRO) specializing in primary cell-based assays. Since 2000, CAS has performed drug toxicity and efficacy testing studies for over 120 pharmaceutical, biotechnology, government, and academic life science organizations worldwide. CAS specializes in the various applications of the in vitro hematopoietic colony-forming unit (CFU) assay, also known as the colony-forming cell (CFC) assay. In addition, CAS performs standardized and customized immunological and mesenchymal cell-based assays. Choose from a portfolio of standardized assays using pre-qualified primary stem cells or discuss your customized needs with our in-house experts. Learn more about Contract Assay Services or discuss your research by contacting contractassay@stemcell.com.
Learn More >Protocols and Resources to Streamline Your Cell-Based Assays
Discover protocols and technical resources to help you work with human primary cells in your research.
Explore More Resources
*Certain products are only available in select territories. Please contact your sales representative or the Product & Scientific Support team at techsupport@stemcell.com for further information.
**Donor Viral Screening Policy
Leukopak, Whole Blood, Purified Cells, Bone Marrow, and LRS Cones - Fresh Products:
Donors are screened for HIV-1, HIV-2, hepatitis B, and hepatitis C. If the donor has been screened within 90 days prior to donation and the results are negative, the product will be shipped with the negative test result and date of the most recent viral testing on the Certificate of Analysis (CoA). If the donor has not been screened within 90 days prior to collection, a test sample will be taken at the time of collection and the product will be shipped before the screening results are available. In the event that a test result is positive, the customer will be contacted as soon as possible (usually within 2 - 4 business days from the time of shipment, and within 4 - 7 business days in the case of fresh LRS Cones).
Leukopak Products, Whole Blood, Purified Cells, and Bone Marrow - Cryopreserved Products:
Donors are screened for HIV-1, HIV-2, hepatitis B, and hepatitis C. If the donor has tested negative within 90 days prior to donation, the product will be shipped with the negative test result and date of the most recent viral testing on the CoA.
Cancer Blood Products - Fresh and Cryopreserved:
Cancer patient donors are screened once initially for HIV-1, HIV-2, hepatitis B, and hepatitis C, with the test date and result recorded on the CoA. Only products with negative test results are shipped.
Cord Blood Products - Cryopreserved Products:
Testing for HIV-1, HIV-2, Hepatitis B, and Hepatitis C is performed on a sample of maternal blood and/ or donated cord blood. Products are shipped with negative test results from the donor screening.
***Fresh Order Fulfillment Policy
Shipment date of fresh leukopak or whole blood orders is subject to change based on the ability of donors to meet procedural requirements during collection or on changes in donor availability. Collections will be rescheduled as soon as possible according to customer requirements.
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.