EasySep™ Release Human CD45 Positive Selection Kit
Immunomagnetic positive selection kit using particle release technology
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration
-
 EasySep™ Magnet
EasySep™ MagnetMagnet for column-free immunomagnetic separation
-
 EasySep™ Buffer
EasySep™ BufferCell separation buffer
-
 Anti-Human CD45 Antibody, Clone HI30
Anti-Human CD45 Antibody, Clone HI30Mouse monoclonal IgG1 antibody against human, chimpanzee CD45
-
Labeling Antibodies
Compatible antibodies for purity assessment of isolated cells
Overview
Desired cells are labeled with antibodies and magnetic particles, and separated without columns using an EasySep™ magnet. Unwanted cells are simply poured off, while desired cells remain in the tube. Then, bound magnetic particles are removed from the EasySep™-isolated CD45+ cells, which are immediately available for downstream applications. Following cell isolation with this EasySep™ Release kit, antibody complexes remain bound to the cell surface and may interact with Brilliant Violet™ antibody conjugates, polyethylene glycol-modified proteins or other chemically related ligands.
Data Figures
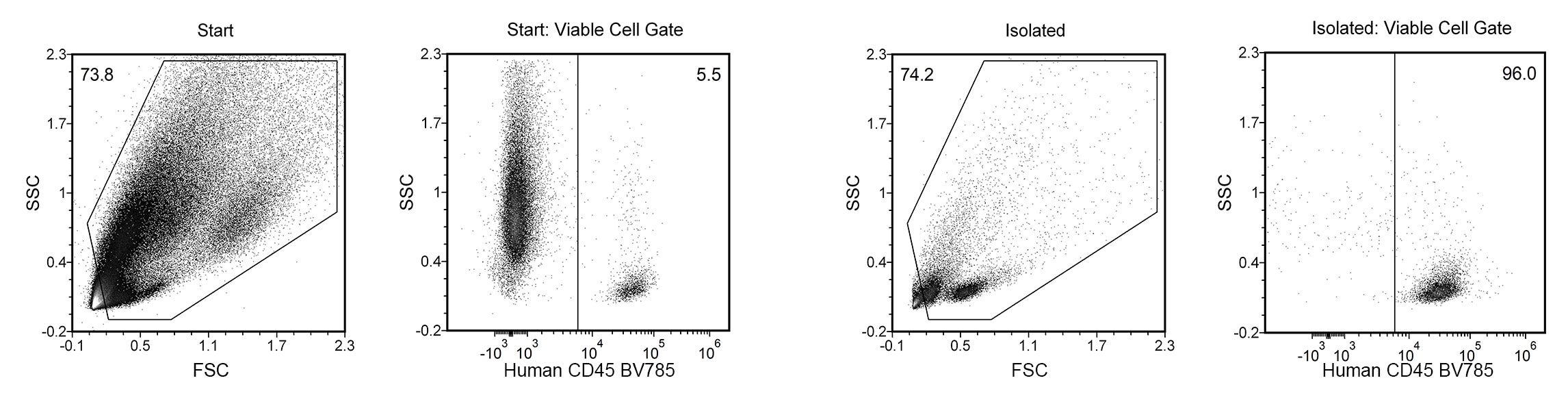
Figure 1. EasySep™ Release Human CD45 Positive Selection Kit
Starting with a single-cell suspension of a human breast cancer tumor xenograft (MDA-MB-231) sample from an NRG-3GS humanized mouse, the CD45+ TIL purities of the start and final isolated fractions are 5.5% and 96.0%, respectively.
NOTE: Cell debris and dead cells were excluded from the analysis based on DRAQ5™ and DAPI fluorescence.
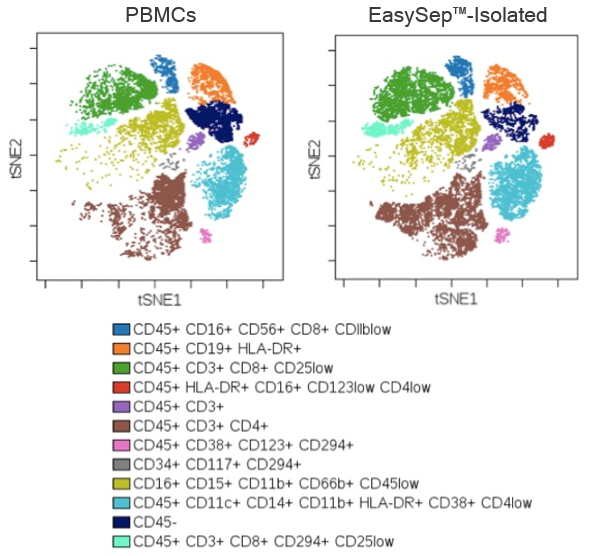
Figure 2. EasySep™-Isolated CD45+ Cells are Representative of the Starting Leukocyte Population
Mass cytometry data comparing the composition of immune subsets in PBMCs and EasySep™-isolated cells from the same donor. Starting with whole blood, PBMCs were prepared by density gradient centrifugation using Lymphoprep™. To compare immune subset composition pre- and post-EasySep™ isolation, a fraction of the PBMCs was further isolated using EasySep™ Release Human CD45 Positive Selection Kit and the pre- and post-isolated fractions were assessed by mass cytometry (CyTOF®). t-SNE plots of cells stained with 19 markers and analyzed by CyTOF® are shown (n = 1).
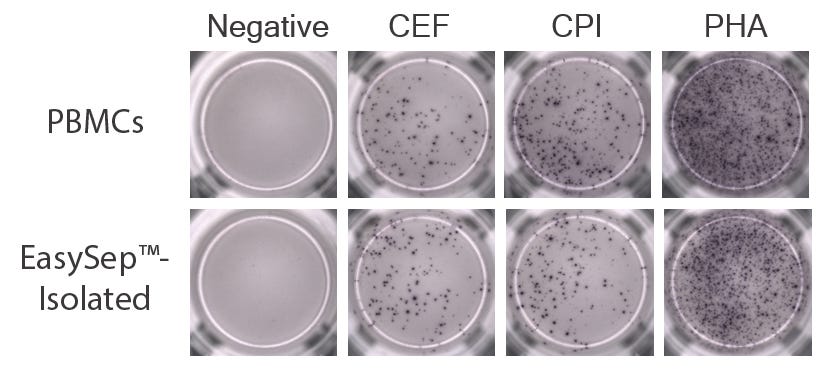
Figure 3. EasySep™-Isolated CD45+ Cells Produce IFN-gamma in Response to Antigen and Mitogen Stimulation
PBMCs pre- and post-EasySep™ isolation were incubated for 24 hours in the presence of peptide pools (CEF for antigen-specific CD8+ T cell response and CPI for antigen-specific CD4+ T cell response) or mitogen (phytohemagglutinin [PHA]). Following incubation, ELISpot plates were processed and IFN-gamma-producing cells were counted using an AID ELISpot reader. Representative images of ELISpot assays are shown (n = 3).
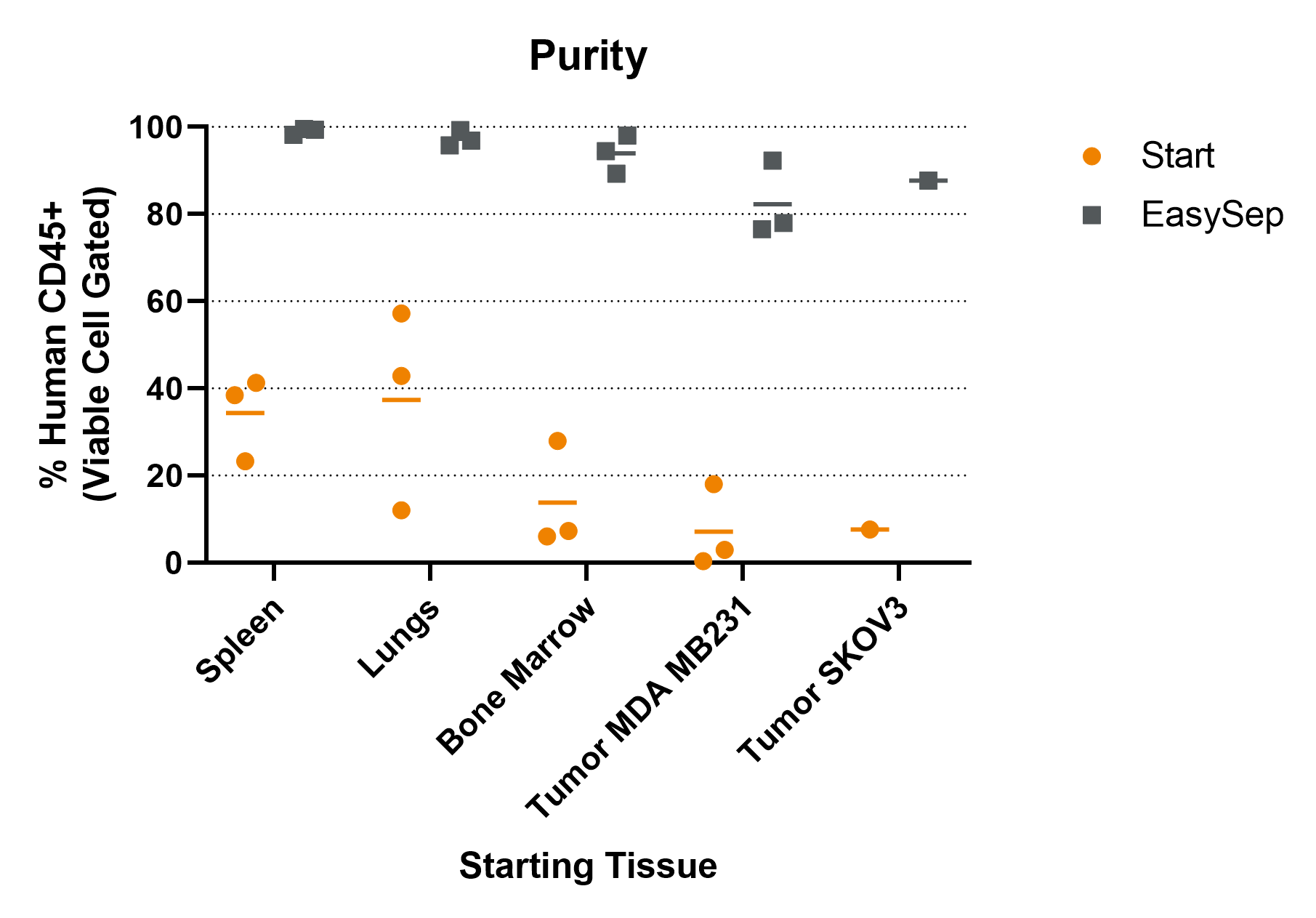
Figure 4. CD45+ Cells Isolated by EasySep™ from Various Tissues Are Highly Purified
A humanized mouse tumor model was generated by engraftment of human CD34+ hematopoietic stem and progenitor cells into NRG-3GS mice followed by xenotransplantation with human cancer cell lines, MDA-MB-231 (breast cancer) or SKOV-3 (ovarian carcinoma). Starting with a single-cell suspension of spleens, lungs, bone marrow, or tumors, human CD45+ leukocytes were isolated using EasySep™ Release Human CD45 Positive Selection Kit. The starting frequencies and isolated purities of human CD45+ cells of individual experiments and averages are shown.
Protocols and Documentation
Find supporting information and directions for use in the Product Information Sheet or explore additional protocols below.
Resources and Publications
Educational Materials (7)
Related Products
Item added to your cart

EasySep™ Release Human CD45 Positive Selection Kit
Quality Statement:
PRODUCTS ARE FOR RESEARCH USE ONLY AND NOT INTENDED FOR HUMAN OR ANIMAL DIAGNOSTIC OR THERAPEUTIC USES UNLESS OTHERWISE STATED. FOR ADDITIONAL INFORMATION ON QUALITY AT STEMCELL, REFER TO WWW.STEMCELL.COM/COMPLIANCE.









