HemaTox™ Kits for In Vitro Hematotoxicity Testing
HemaTox™ Media and Supplements for In Vitro Hematotoxicity Testing
Why Use HemaTox™ Kits?
- Tested in comparison to the appropriate CFU assay in IC50 determination, the current standard for in vitro IC50 determination.
- Optimized serum-free medium and supplement provide robust expansion, allowing consistent results.
- Test compounds may be added at the start of culture to examine effects on progenitors before differentiation or later during culture to study effects on more differentiated cells. Different readout methods may be used, e.g., flow cytometry.
Data

Figure 1. General HemaTox™ Kit Procedure
*The cell isolation step may be omitted if pre-enriched CD34+ cells are used.

Figure 2. Flow Cytometry Plots Showing Cells Produced After Culture of CD34+ HSPCs with HemaTox™ Erythroid, Myeloid and Megakaryocyte Kits
(A) Human CB CD34+ cells were cultured with (B) HemaTox™ Erythroid, (C) Myeloid and (D) Megakaryocyte Kits using the protocol above. After the appropriate culture period, cells were harvested and stained for cell surface proteins expressed on erythroid (CD71 and GlyA), myeloid (CD13 and CD15) or megakaryocytic (CD45 and CD41) cells, respectively.
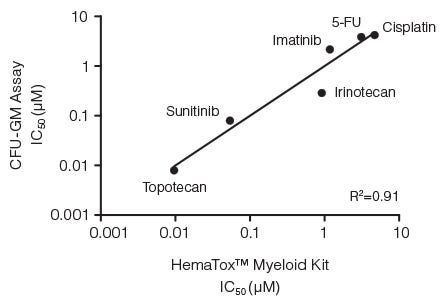
Figure 3. Correlation Between IC50 Values for Six Drugs Measured Using the CFU-GM Assay and the 96-Well Plate Liquid Culture-Based HemaTox™ Myeloid Kit
Human BM CD34+ cells were cultured in colony-forming unit - granulocyte/macrophage (CFU-GM) assays with MethoCult™ medium and in liquid culture with the HemaTox™ Myeloid Kit. IC50 values generated using each assay were plotted on the X and Y axes and shown to be highly correlated with a coefficient of determination (R2) of 0.91.

Figure 4. Lineage-Specific Differences in Hematotoxicity Identified with HemaTox™ Erythroid, Myeloid and Megakaryocyte Kits
Results show average IC50 values for each drug tested on human BM CD34+ cells using the HemaTox™ Erythroid (grey), Myeloid (gold) and Megakaryocyte (orange) Kits. Most drugs show similar toxicity for each lineage but some, such as Sunitinib, are ~100-fold more toxic for erythroid than for megakaryocyte progenitor differentiation with intermediate toxicity for myeloid progenitor differentiation. Vertical lines indicate standard error of the mean (SEM) (n = 4 - 8).
Contract Assay Services
Engaging the right expertise and resources can be an effective way to create efficiencies in your drug development pipeline. Our Contract Assay Services (CAS) team combines optimized media, pre-qualified cells, and specialized expertise to screen candidate therapeutics in primary cell-based assays.