Organoids as Models of Infectious Disease
Questions and Answers from the Mini-Symposium
During our mini-symposium on Organoids as Models of Disease, co-hosted with Hubrecht Organoid Technology (The HUB), we asked the audience for their questions related to organoid cultures and the applications of organoids in studying health and disease. Below, scientists from The HUB answer some of the questions we didn't have time to address during the symposium.
Organoids in Cancer
How are organoids being applied to study cancer? What types of cancer can be modeled with organoids?
Patient‑derived tumor organoids can be generated from most carcinomas and are commonly generated from colon, breast, lung, pancreas, ovarian, head, neck, and bladder tumors. These organoids can be expanded in culture, banked, or used in molecular applications, including genomics, transcriptomics, and proteomics. Recent studies have shown differences in efficacy of drugs tested in 2D- versus 3D‑cell cultures, patient‑derived tumor organoids can serve as a powerful in vitro system for drug screening and development as they represent the in vivo tumor environment better than immortalized cell lines.
How are tumor‑derived organoids established? What considerations should I make when trying to establish tumor‑derived organoids?
Tumor‑derived organoids can be established using the same or a similar approach to organoids derived from normal/healthy tissue: Organoids are established by isolating cells from tumor biopsies or from resected tissue and resuspending these cells in extracellular matrix (ECM) such as Matrigel® or Cultrex®. Organoids spontaneously self‑organize from the resuspended tumor cells with medium changes performed every 2‑3 days and culture passages performed every 7‑10 days. It is important to use ROCK inhibitor during the initial culture to avoid single cells entering Anoikis.
Can organoids be established from circulating tumor cells? How can I use organoids to expand rare cancer cell types?
Organoid cultures have been established from resected tissue, core needle biopsies, and fine needle biopsies. Therefore, researchers should be able to establish organoid cultures from liquid biopsies as well. Important factors that can impact the success of establishing these cultures are tumor cell content and the proliferative capacity of the tumor cells themselves. Organoid culture media designed for expanding healthy adult stem cells is generally amenable to culturing tumor‑derived organoids and is non-selective, allowing for growth of rare cancer cell types.
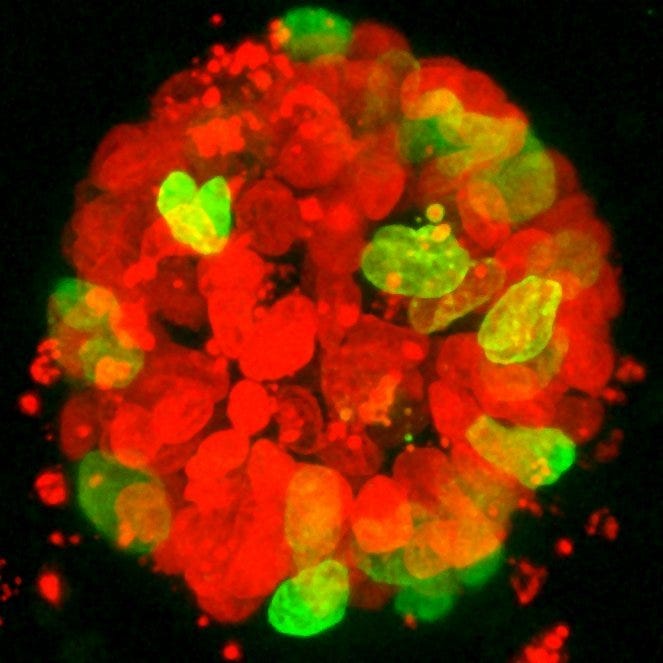
A colon tumor organoid. Tumor cells show expression of recombinant H2B-NeonGreen (Green), cell nuclei are labelled with DAPI (red).
How are organoids being used to develop new cancer therapies? How do treatments like radiation or other commonly used treatment strategies affect organoid integrity?
Organoids have been shown to accurately predict the response to treatment in a variety of cancer- and tumor‑types (Vlachogiannis et al., 2018; Sachs et al., 2018; Yao et al., 2020; Ooft et al., 2019). This makes patient‑derived organoids (PDOs) very powerful preclinical platforms for drug screening and drug development in a human- or even patient‑specific context. Multiple groups have used PDOs in large screens and have shown high levels of reproducibility with the typical response to treatment strategies being inhibition of proliferation or organoid death. However, it should be noted that the response will vary by treatment type, dosage, organoid type, and by donor.
How have organoids been used in immuno‑oncology studies? What implications does this have for the potential of applications in precision medicine (eg. endocytosis assays to study immunoconjugate‑mediated receptor internalization)?
Organoids have increasingly been adopted by the immuno‑oncology field as a way to study the interaction of the immune system with epithelial tumors. However, robust systems to enable tumor organoid‑immune cell co‑culture are still in development. Recent advances using leukocytes and T cells to examine retention, activation, and organoid cytolysis have shown that organoids are able to model the interactions observed in vivo. This has enabled researchers to effectively evaluate the cytotoxicity of a variety of antibody‑drug‑conjugates (e.g. trastuzumab, emtansine, neratinib) in vitro.
How can organoids be co‑cultured with other cell types to better recapitulate the tumor microenvironment (eg. co‑culture with haematopoietic cell types)?
Co‑culturing organoids with other cell types is a major challenge currently being addressed by the field. Ideally, the best way to recapitulate the tumor microenvironment would be to grow cancer organoids cultured directly from tumour resections while preserving endogenous immune and other non‑epithelial cells. However, current organoid culture technologies only allow for the expansion of the epithelial compartment of the tumor.
One strategy to recapitulate additional cellular diversity is to expand each desired cell-type independently until sufficient material is available for performing your assay, then reconstituting these different cell fractions with primary organoids. Protocols that preserve the cellular diversity found in primary organoids for prolonged periods are currently under development. For example, Neal et al. (Cell 2018) integrated epithelial and stromal compartments that allowed preservation of hematopoietic cell types.
Organoids in Viral Studies
How are organoids being used to model viral infections?
Organoids have been used to study viral propagation, viral replication, and host responses to viral infection. Organoid cultures can serve as a platform to study and screen antiviral drugs and offer the opportunity to explore patient‑specific responses to both infection and treatment.
For example, Lamers et al. (2020) recently published a proof‑of‑concept study showing that the novel SARS‑Coronavirus‑2 can replicate in intestinal organoids. Other examples include the use of human airway organoids infected with Respiratory Syncytial Virus to recapitulate central disease features (Sachs et al., 2019) and offer a model to study the infectivity of emerging influenza strains (Zhou et al., 2018).
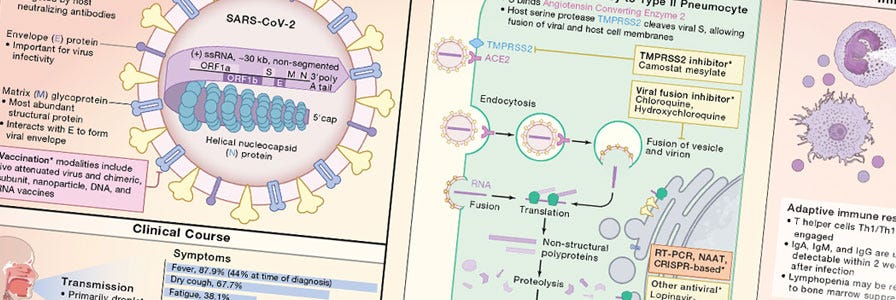
What role have airway organoids played in modeling and understanding SARS‑CoV‑2 infection and pathogenesis?
Airway organoids are playing a major role in unveiling the mechanics of the host‑virus interaction. Thanks to their ability to recapitulate the cellular diversity of the airway epithelium, airway organoids are serving as instrumental models for identifying potenial COVID‑19 therapeutics. Studies using airway organoids confirmed that SARS‑CoV‑2 infection is directly dependent on the expression of the ACE2 receptor at the cell surface of host cells and that auxiliary cell membrane‑bound proteases can facilitate viral entry. Additionally, infecting lung organoid cultures with SARS‑CoV‑2 has provided information on the preferred target cell type revealing higher susceptibility to infection of ciliated cells compared to goblet cells. Lastly, using human pluripotent stem cell‑derived organoids researchers were able to show how SARS‑CoV‑2 infection induces chemokine production similar to what has been observed in COVID‑19 patients.
Cell Snapshot: COVID‑19
Request your copy of the COVID‑19 wallchart featuring a useful overview of the viral life cycle and human immune response to SARS‑CoV‑2.
What (if any) studies have been done on SARS‑CoV‑2 using kidney organoids? How might kidney organoids be co‑cultured with SARS‑CoV‑2 and immune cells to model infection?
The full extent to which SARS‑CoV‑2 infects organs other than the lungs is still under investigation. Renal abnormalities have been observed in COVID‑19 patients, and podocytes and tubular epithelial cells have been shown to be susceptible to SARS‑CoV‑2 infection. The Penninger lab was among the first to use differentiated human kidney organoids to model SARS‑CoV‑2 infection in the kidney. However, further investigation will be required to better understand the role of the immune system in combating the disease. Understanding the factors that contribute to the considerable variability that has been observed between patients will be especially important in establishing effective treatment options. Co‑culturing kidney organoids with allogeneic immune cells is presenting itself as a fruitful avenue of investigation for better understanding the pathophysiology of SARS‑CoV‑2 and for elucidating the factors contributing to the variability in different patient’s response to infection.
How are GI organoids (including organoid‑derived monolayers) being used to study bacterial adhesion in the GI tract? What protocols are available for applying this model in my own research?
The use of bacterial co‑culture with intestinal organoids has dramatically increased given the importance of the gut microbiota on human health and disease. For example, bacterial‑organoid co‑cultures were used to identify the mutational signature caused by prolonged exposure to genotoxic E. coli. However, different experimental approaches and culture methods are needed if co‑cultures are being maintained in the short- or long‑term.
To model short‑term interactions, organoid‑derived monolayers provide a useful system as they enable easy access to the apical side of the epithelium. By culturing these monolayers at the air‑liquid interface, intestinal monolayers undergo further morphological changes that closely mimic in vivo intestine, providing a flexible platform for the investigation of bacterial pathogenesis (Moon et al., 2014; Pott et al., 2018). However, for long‑term studies, cultures microinjected with bacteria are better maintained in 3D (Pleguezuelos‑Manzano et al., 2020) as this enables longer proliferation of the epithelial cells in culture.
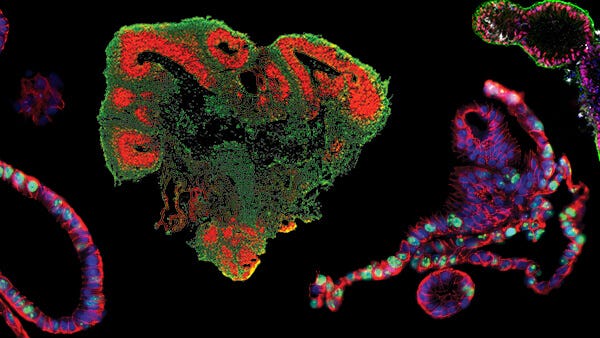
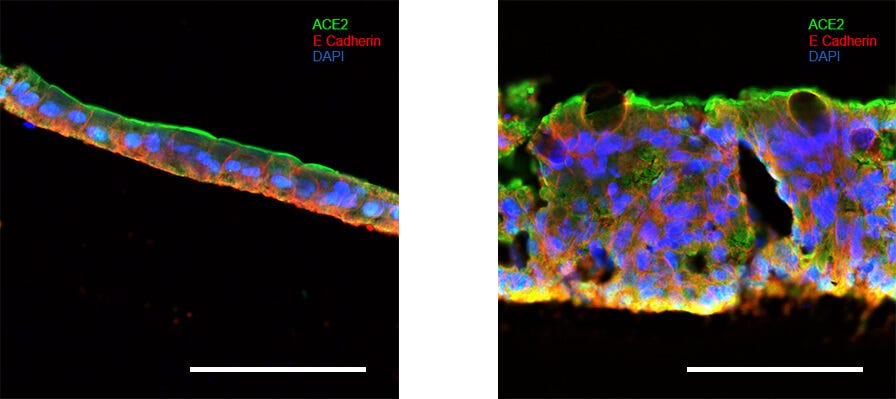
Intestinal organoids can be used to investigate SARS‑CoV‑2 infection of the intestinal epithelium and pathogenesis of the virus in the gut. Above, intestinal organoids were grown in IntestiCult™ Organoid Growth Medium (Human) and used to seed organoid-derived monolayers. Immunofluorescence staining of (Left) organoid-derived submerged monolayers and (Right) organoid-derived monolayers cultured at the ALI show strong staining for ACE2 at their apical surface. Scale bars = 100 μm.
How can organoids be used in a high‑throughput manner for viral studies? What considerations need to be made for both organoid initiation and assay development in order to support the high‑throughput application of airway organoids for studying SARS‑CoV‑2 morbidity and mortality?
One key consideration is whether the viral infection occurs via the apical or basolateral side of the cell. Viruses that require access to the apical side require micro‑injection into the lumenal compartment of the organoids, or culture as monolayers, enabling access to the apical surface. Viruses that infect via the basolateral side can employ either monolayers cultured on a Transwell® insert or organoids cultured in 3D. The experimental readout is another important consideration. For example, if measuring the effect of the virus or a potential treatment on the epithelium, cell death can be a useful output as the organoids recapitulate the effects of viral infection and cell death. In contrast, by co‑culturing with immune cells, immune cell activation can be an effective readout. However, organoids can only provide an understanding of the viral infection in a tissue‑specific context and cannot inform on patient morbidity or mortality.
Organoids in Drug Development
How are organoids affecting the landscape of clinical and preclinical drug development? How might this change in the future? What are the challenges of current organoid systems that need to be overcome to realize this?
Organoids are already being used to assess the efficacy and toxicity of lead compounds. The use of 3D cell culture models in this application is important as 2D cell culture systems do not accurately represent how a drug candidate affects cells in a 3D system. There are many examples of therapeutic candidates that have shown promise in 2D cultures only to fail when applied to a 3D system. Identifying the efficacy of lead compounds will reduce the time needed to validate compounds and will reduce the overall cost as more compounds will be screened out earlier in the development pipeline. As organoids respond to therapeutic compounds in the same manner as in vivo, they can be used to predict clinical response and can therefore be used to tailor the patient population of clinical trials and as companion diagnostic tools. The main challenges currently faced by the field is reducing the time required to generate a sufficiently large pool of organoids from a biopsy to perform a diagnostic screen and the efficient culture of large numbers of organoids for large scale screening.
Can organoids be used to predict the side effects of potential therapeutics?
Organoids have been used in toxicology studies to predict the cytotoxicity of certain compounds to the healthy tissue. Organoids offer a more physiologically relevant model for understanding of the mechanism behind the therapeutic activity of the compound enabling a better prediction of the effects on healthy and diseased tissues.
Intestinal organoids can be greatly expanded to provide large scale platforms for screening. Above, organoids are labeled with the nucleotide analog EdU (green) and DAPI (red). Newly dividing cells are seen in green as they incorporate the nucleotide analog into their replicating DNA.
Other than Matrigel®, what cell matrices can be used for culturing organoids? Are there more defined matrices available?
The extracellular matrix (ECM) is a key component of organoid culture as it is the scaffold that enables organoids to grow in three dimensions. The most common ECMs used for organoid culture are Matrigel® and Cultrex®, both generated from the mouse Engelbreth-Holm-Swarm (EHS) tumor. These ECMs enable the growth of organoids from a wide range of normal and diseased samples from nearly all tissues. However, new approaches are employing tissue‑specific scaffolds, which might contain tissue‑specific ECM composition or other regulatory factors. Alternatively, fully‑defined synthetic matrices are better suited for research into clinical and therapeutic applications than their animal‑derived counterparts (Gjorevski et al., Nature 2016).
Organoids as Model Systems
What are the advantages of organoids over other ex vivo models? What are the challenges, or other considerations, associated with adopting an organoid model system?
Organoids can be generated from epithelial tissue of any species and can be generated from a variety of organs including intestine, liver, pancreas, breast, and lung from both healthy and diseased tissue (e.g., cancer), or from differentiated pluripotent stem cells. Orgaonids form a tissue‑like structure that histologically is very similar to the actual organ. Organoid cultures have long term expansion capacity, are untransformed, genetically and phenotypically stable, and retain biological and functional properties of the original tissue. Epithelial stem cells in the organoids support their expansion and at the same time differentiate to all cell types present in the tissue providing a heterogeneous microenvironment. Cultures can be tailored and scaled up as needed for high‑throughput screening, providing a unique and robust in vitro model for drug development, diagnosis, and patient stratification.
One of the biggest challenges of organoid culture is its current dependency on animal‑derived extracellular matrices (ECMs) such as Matrigel®. Synthetic matrices such as hybrid polyethylene glycol‑hydrogels or micro‑engineered collagen scaffolds, combined with a well‑defined set of laminins, may better fulfil the niche requirements of organoids and may be able to be customized for a specific tissue or application. The future success of autologous transplantation of genetically repaired or in vitro expanded organoids in patients is dependent on a significant improvement in engraftment efficiency which may be helped by the development of novel ECMs.
Although organoids recapitulate the tissue of origin with high fidelity, most organoids lack the other cell types present in the tissue (e.g, endothelial cells, immune cells, stromal cells), providing boundaries of what organoids can model in vitro. Organ‑on‑a‑chip technologies may in part overcome this limitation, as many have successfully combined multiple cell types to effectively model aspects of human physiology.
What are your key recommendations for a new investigator interested in starting organoids in their lab for the first time?
Organoid culture does not have to be complex, however, it should be thoroughly considered whether organoids or another system (e.g. spheroid, 2D culture, animal models) are the most appropriate system for the question at hand. If organoids are the best system for the intended application, it’s important to determine whether tissue‑derived or pluripotent stem cell- (PSC-) derived organoids should be used. Factors influencing this decision often include access to primary tissues and/or experience culturing PSCs.
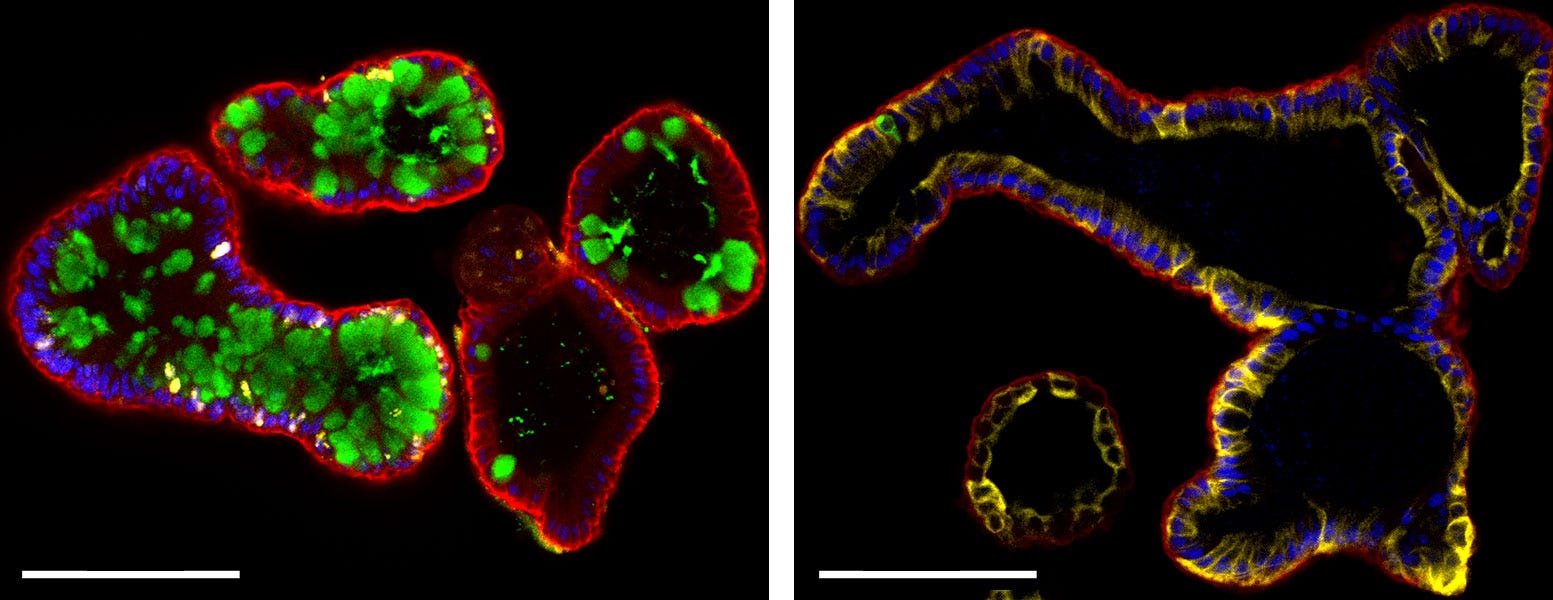
Differentiated intestinal organoids contain cell‑types of the mature intestinal epithelium. (Left) Intestinal organoids differentiated in IntestiCult™ Organoid Differentiation Medium contain cells of the mature intestinal epithelium including goblet cells (Muc2; left, green), enterocytes (Krt20; right, yellow), and enteroendocrine cells (ChgA; right, green), while maintaining an actively dividing stem cell population (ki67; left, yellow). Organoids are also stained for E-Cadherin (both; red) and cell nuclei (DAPI; blue). Scale bars = 100 μm.
What is needed next in the development of organoid model systems? How do we make them even more representative of organ physiology?
Much investigation remains to fully understand to what extent epithelial organoids can reproduce the function of healthy and disease tissue. However, as many of these responses depend on other cell types, there is interest in culturing organoids with non‑epithelial cell types. For example, many immuno‑oncology groups are attempting to study T Cell response to different treatments when co‑cultured with healthy tissue- or tumor‑derived organoids. In other cases, organoids have been used to develop organ‑on‑a‑chip platforms that combine multiple tissue and cell types (e.g., epithelium, endothelium, immune cells), enabling a greater understanding of how these systems interact.
What balance is needed between complexity and simplicity in organotypic model systems? How do we achieve this balance as we continue to develop more physiologically representative model systems?
The ideal model system balances complexity and relevancy for the question at hand. Model systems range in complexity from simple, immortalized 2D cell culture, to 3D organoid models, to complex animal models, each of which is optimally relevant for a different set of biological questions.
For example, cell lines are simple systems that are maximally relevant for investigating basic cellular biological questions and only minimally recapitulate the necessary complexity of diseased states seen in a clinical setting. Similarly, animal models provide the multi-system complexity seen in human subjects, but fundamental physiological differences can limit their relevance when trying to understand the human state. It is imperative then that researchers bear in mind the complexity offered by their choice of model system and its relevance to the question being addressed.
Recent studies have shown that patient‑derived organoids from several cancer types (e.g., colorectal cancer, pancreatic cancer, breast cancer) and several genetic diseases, such as Cystic Fibrosis, are able to recapitulate the response seen in the patient, indicating that organoids can be used to accurately model treatment outcomes.
As more complex models emerge the balance between complexity and relevancy will be ever more important; the increase in biological complexity will necessitate an equivalent increase in physiological relevance, which will need validation alongside clinical data.
Research Roundtable
Read key insights and watch the webinars from the Nature Reseach Roundtable on Organoids.
Growing Organoids Wallchart
Decorate your lab with a handy overview of epithelial organoid systems.
Organoid Resource Center
Find additional organoid webinars, technical resources, researcher profiles, and more.