Air-Liquid Interface Culture for Respiratory Research
.
- Document # 29885
- Version 2.0.0
- Jul 2019
The Human Tracheobronchial Epithelium
The epithelial lining of the tracheobronchial region of the human respiratory tract is classified as a pseudostratified, ciliated, columnar epithelium. Three cell types predominate in this region: ciliated cells, secretory cells (primarily mucus-secreting goblet cells) and basal cells.1
The tracheobronchial epithelium acts as a protective barrier, defending against a variety of inhaled insults such as toxins, pollutants and pathogens. Barrier function is maintained primarily as a result of three key characteristics of the epithelium:
- Tight junction proteins maintain the integrity of the epithelium and are critical to its function as a physical barrier.
- Secreted mucus traps particulate matter and pathogens, which are subsequently transported out of the airway by the coordinated action of ciliated cells, in what is known as the mucociliary escalator.2,3
- Secreted protective mediators such as antimicrobial peptides and inflammatory mediators form a chemical and immunological barrier against pathogens and toxicants.4 There is a growing need for physiologically relevant models of the respiratory epithelium, but recreating these complex functions in vitro is a considerable challenge.
Modeling the Respiratory Epithelium In Vitro
A variety of cell types can be used to model the human respiratory system, including immortalized respiratory cell lines (e.g. BEAS- 2B and 16HBE14o- cells) and primary cells from animals or human donors. Although the use of immortalized cell lines and primary cells from animals is common, data generated using these models are not directly applicable to the human system.5 Submerged culture of primary human bronchial epithelial cells is possible; however, cells in this system fail to undergo mucociliary differentiation. In order to recapitulate the pseudostratified mucociliary phenotype observed in vivo, primary human bronchial epithelial cells (HBECs) must be cultured at the air-liquid interface (ALI; Figure 1).6
The defining feature of ALI culture is that the basal surface of the cells is in contact with liquid culture medium, whereas the apical surface is exposed to air. A common approach is to seed cells onto the permeable membrane of a cell culture insert, which is initially supplied with culture medium to both the apical and basal compartments (Figure 1A). Once confluence is reached, the cells are subjected to ‘air-lift’, where the medium is supplied only to the basal chamber (Figure 1B). This configuration mimics the conditions found in the human airway and drives differentiation towards a mucociliary phenotype.
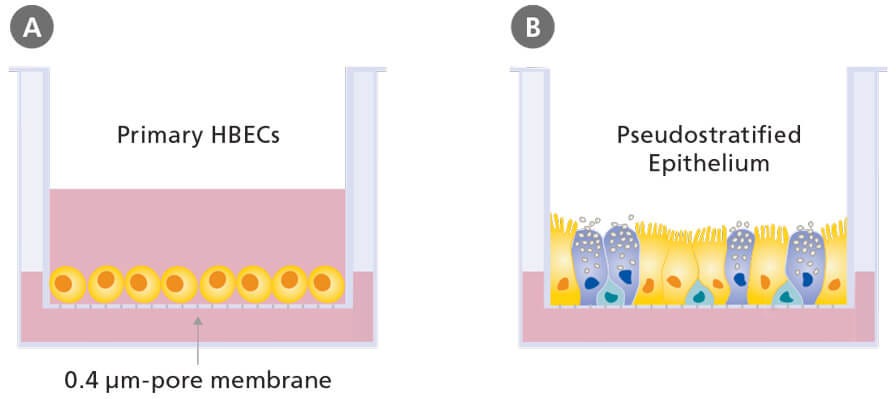
Figure 1. Air-Liquid Interface Culture Schematic
Schematic of submerged (A) and air-liquid interface (B) culture of primary human bronchial epithelial cells grown using porous culture inserts.
Physiological Relevance of Air-Liquid Interface Culture
ALI culture of primary HBECs is increasingly being recognized as an important culture system that facilitates physiologically relevant respiratory research.5 HBECs cultured at the ALI undergo extensive mucociliary differentiation, resulting in an in vitro model that is representative of the in vivo airway. Hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining show that ALI cultures (Figure 2A and 2C), like the in vivo bronchial epithelium (Figure 2B and 2D), are pseudostratified in morphology and are made up of a heterogeneous cell population, including ciliated and mucus-secreting (PAS-positive) cells.
This model is also characterized by the development of epithelial barrier function, as indicated by the expression of tight junction proteins and the development of high transepithelial electrical resistance.6 The suitability of ALI cultures for modeling the airway has been further confirmed through transcriptome analyses7 and a wide range of experiments demonstrating physiological responses to insults such as toxicants and pathogens.8–10 Furthermore, ALI cultures of primary cells from donors with respiratory disease (e.g. asthma, cystic fibrosis, COPD) recapitulate in vivo disease characteristics, forming robust in vitro models of these conditions.11,12
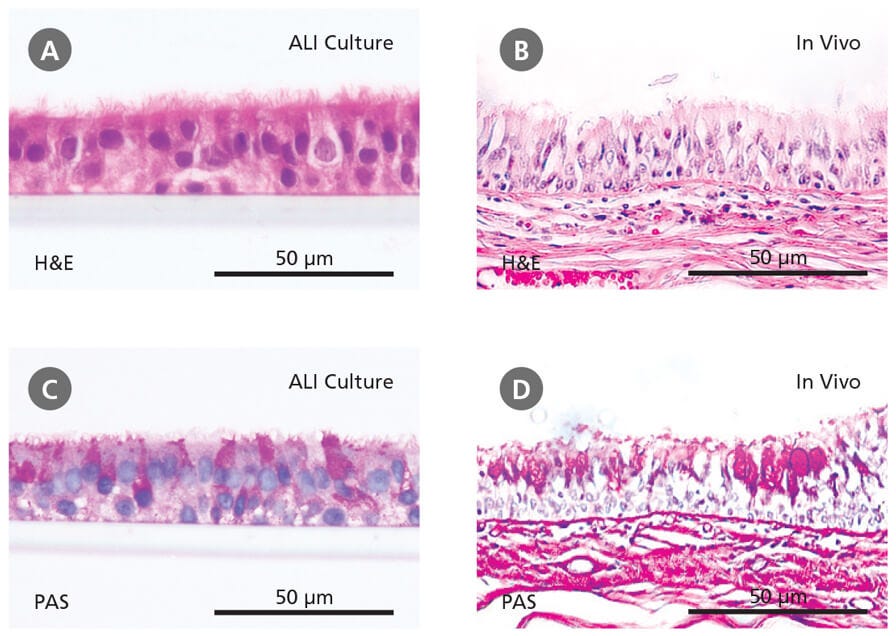
Figure 2. Primary Human Bronchial Epithelial Cells Cultured at the Air-Liquid Interface Recapitulate the In Vivo Bronchial Epithelium
(A-B) Hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining reveals that cells differentiated at the air-liquid interface in PneumaCult™-ALI (A and C) form a pseudostratified epithelium that is representative of the in vivo bronchial epithelium (B and D). Data in (A) and (C) generated by Samuel Wadsworth.
PneumaCult™-ALI for Air-Liquid Interface Culture
PneumaCult™-ALI (Catalog #05001) is a serum- and BPE-free culture medium that has been optimized to reproducibly support improved mucociliary differentiation of primary HBECs in ALI cultures.
Applications of Air-Liquid Interface Culture
Air-liquid interface culture holds enormous potential for respiratory research, with a wide range of experimental uses. This includes a number of highly specialized applications for which submerged culture techniques provide inadequate models. Use ALI cultures of airway epithelial cells to:
- Study the cell biology of the respiratory epithelium
ALI cultures are the most physiologically relevant model for studying the respiratory epithelium in vitro.6 - Model respiratory diseases
Airway epithelial cells from patients with chronic respiratory diseases such as cystic fibrosis, COPD and asthma, can be cultured using ALI techniques, enabling disease mechanisms to be studied in vitro.11,12 - Study infection of the respiratory epithelium
Some respiratory viruses selectively target cell types present only in fully differentiated airway cell cultures.13,14 - Test drug formulations for inhalation delivery
Aerosol particles can be directly deposited onto the semi-dry apical cell surface, mimicking the deposition of powders onto the lung surface in vivo.15,16 - Test toxicity of inhaled substances
Responses of ALI-differentiated primary epithelial cells to insults such as tobacco smoke components closely mimic reported changes in the human airway.17,18
Featured Content and Resources
Respiratory System Overview
Get a free wallchart providing an organizational and functional overview of the respiratory system.
Optimizing In Vitro Lung Models
Dr. Rachael Rayner compares the effects of various expansion and differentiation media on primary airway cells cultured at the ALI.
References
- Ehrhardt C, et al. Drug Absorption Studies II: 235-257, 2008
- Fahy J V and Dickey BF. New Engl J Med 363(23): 2233-2247, 2010
- Button B, et al. Science 337(6097): 937-941, 2012
- Tam A, et al. Ther Adv Respir Dis 5(4): 255-273, 2011
- Bérubé K, et al. Toxicology 278(3): 311-318, 2010
- Prytherch Z, et al. Macromol Biosci 11(11): 1467-1477, 2011
- Dvorak A, et al. Am J Respir Cell Mol Biol 44(4): 465-473, 2011
- Thaikoottathil J V, et al. Eur Respir J 33(4): 835-843, 2009
- Krunkosky TM, et al. Microb Pathog 42(2-3): 98-103, 2007
- Palermo LM, et al. J Virol 83(13): 6900-6908, 2009
- Ostrowski LE, et al. Lung 190(5): 563-571, 2012
- Comer D, et al. Eur Respir J doi: 10.1183/09031936.00063112
- Matrosovich, MN et al. Proc Natl Acad Sci U S A 101(13): 4620-4, 2004
- Hao W, et al. J Virol 86(24):13524-13532, 2012
- Lin H, et al. J Pharm Sci 96(2): 341-350, 2007
- Tralau T and Luch A. Trends Pharmacol Sci 33(7): 353-364, 2012
- Andreoli C, et al. Toxicol in Vitro 17(5-6): 587-594, 2003
- Balharry D, et al. Toxicology 244(1): 66-76, 2008
Request Pricing
Thank you for your interest in this product. Please provide us with your contact information and your local representative will contact you with a customized quote. Where appropriate, they can also assist you with a(n):
Estimated delivery time for your area
Product sample or exclusive offer
In-lab demonstration