T Cell Therapy
Your breakthrough today can become the therapy of tomorrow. That’s why we develop reagents and protocols to enable T cell therapy research and manufacturing.
The science is beautifully complex. The most well-known form of T cell therapy, chimeric antigen receptor (CAR) T cells, are engineered to express synthetic transmembrane receptors to redirect their specificity toward cancer cells. Read our mini-review on CAR T cell research and wallchart on CAR T cell production for more information:
Production of CAR T Cells Wallchart
Free Nature Protocols Wallchart summarizing the processes involved in producing CAR T cells for therapy.
Immunology Feature: CAR T Cells
A mini-review of our selection of key advancements in CAR T cell therapy research.
Reagents for T Cell Therapy Research
Production of human T cells for cell therapy is a complex, multi-step process. There are many opportunities for optimization to obtain maximum yield while retaining desired end phenotype and function. Explore optimized protocols and reagents for human T cell research:
T Cell Expansion
Expand human T cells without the use of serum in cGMP-manufactured media.
GloCell™ Fixable Viability Dyes
Easily validate cell viability in applications such as flow cytometry.
T Cell Analysis
Evaluate with antibodies and ELISA kits compatible with our cell isolation and cell culture products.
See the data below and try our T cell isolation, activation, and expansion reagents in your own lab.
Read these Technical Bulletins and optimize your human T cell protocols using these reagents.
Optimization of Human T Cell Expansion Protocol: Effects of Early Cell Dilution
This Technical Bulletin demonstrates how maintaining T cells at lower cell densities can improve T cell growth and viability, especially at early stages of cell expansion.
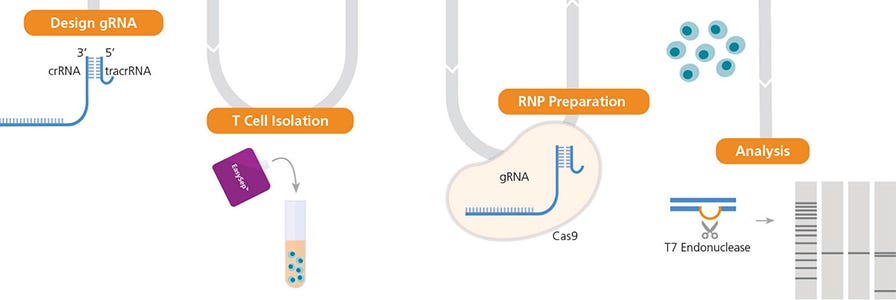
High-Efficiency Genome Editing of Human Primary T Cells
This optimized and validated protocol details how to genetically edit T cells with high efficiency using the ArciTect™ CRISPR-Cas9 System. Our experiments highlight important pre- and post-editing culture considerations for robust genome editing and cell expansion.
Why Use STEMCELL’s Reagents for Cell Therapy Research Applications?
- Traceability documentation including CoAs and CoOs to help reduce time in preparing IND submissions or clinical trial applications.
- Defined formulations to minimize lot-to-lot variability.
- Extensive QC testing.
- Experienced global professionals to help navigate regulatory processes.
We can currently work with you to qualify these reagents under an approved investigational new drug (IND) or clinical trial application (CTA). Read this technical bulletin or watch this webinar to learn about the fundamentals of ancillary material qualification.
Contact us for more information on how we can support your cellular therapy research.
Data
T cells isolated using EasySep™ Release Human CD3 Positive Selection Kit are highly purified.
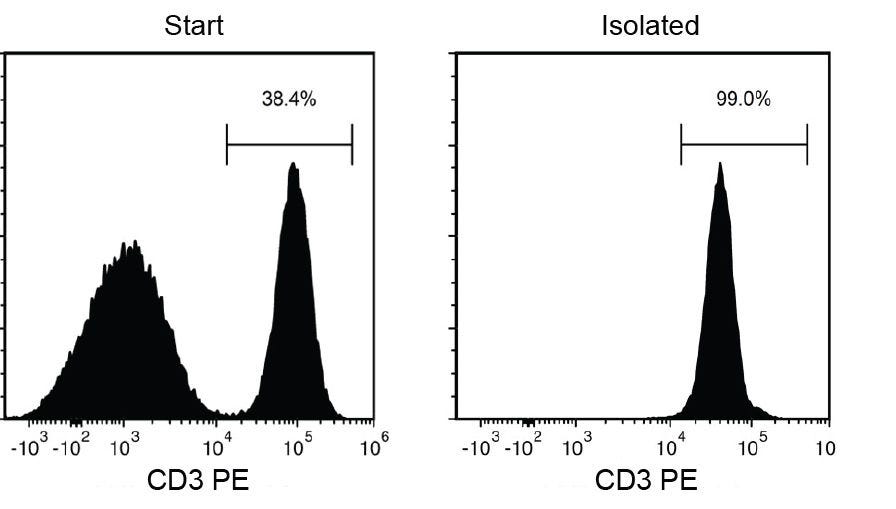
Figure 1. Typical EasySep™ Release Human CD3 Positive Selection.
Starting with a single-cell suspension of human PBMCs, the CD3+ cell content of the isolated fraction is typically 98.7 ± 0.9% (mean ± SD using the purple EasySep™ Magnet). In the above example, the purities of the start and final isolated fractions are 38.4% and 99.0%, respectively.
T cells express activated phenotype and morphology when stimulated with ImmunoCult™ T Cell Activators in ImmunoCult™-XF T Cell Expansion Medium.
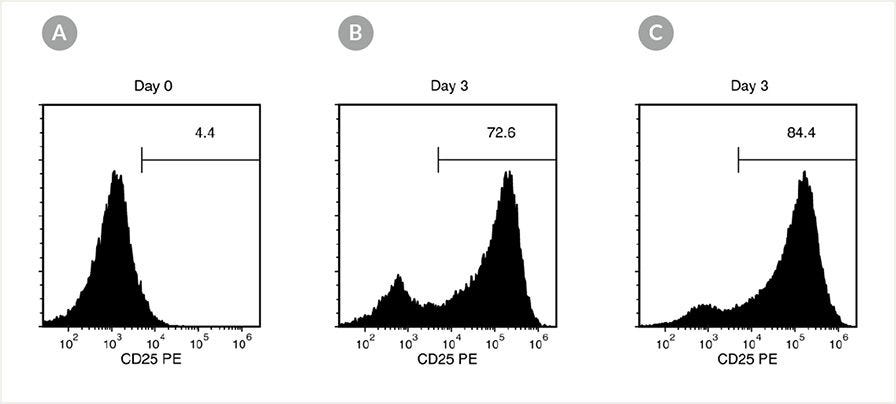
Figure 2. T cells are activated when stimulated with ImmunoCult™ Human CD3/CD28 or CD3/CD28/CD2 T Cell Activator.
T cells were cultured on day 0 with either ImmunoCult™ Human CD3/CD28 T Cell Activator (Catalog #10971) or ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator (Catalog #10970) in ImmunoCult™-XF T Cell Expansion Medium (Catalog #10981). Cells were gated on CD4+ T cells and CD8+ T cells and T cell activation was accessed by CD25+ expression on day 0 and day 3. At the start of culture, the CD25+ cell population was (A) 5.63 ± 2.4% (mean ± SD). After three days of activation, the CD25+ cell population was (B) 75.4 ± 13.8% (mean ± SD) when activated with ImmunoCult™ Human CD3/CD28 T Cell Activator and (C) 88.8 ± 3.2% (mean ± SD) when activated with ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator.
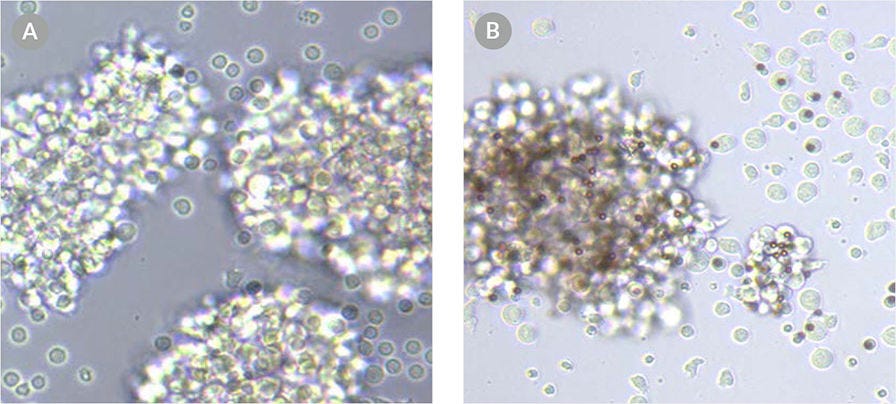
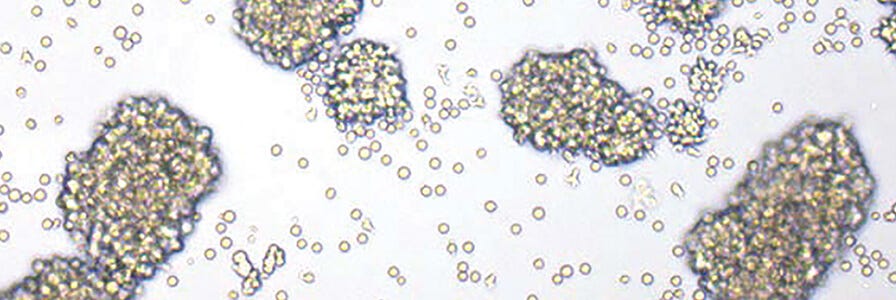
Figure 3. T cells exhibit an activated morphology when activated with ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator.
Images of T cells activated with (A) soluble ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator and (B) the competitor's bead-based activation reagent.
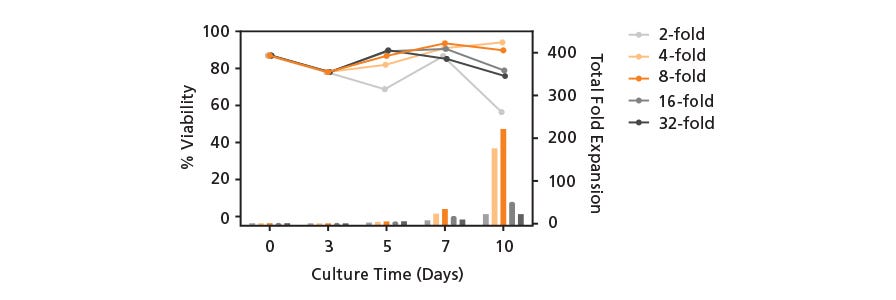
T cells show robust fold expansion and high viability when expanded with ImmunoCult™ T Cell Activators in ImmunoCult™-XF T Cell Expansion Medium.
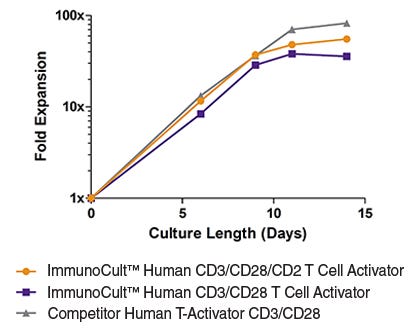
Figure 4. T cells show robust expansion when stimulated with ImmunoCult™ Human T Cell Activators in ImmunoCult™-XF T Cell Expansion Medium.
T cells were expanded over 14 days with ImmunoCult™ Human CD3/CD28 T Cell Activator, ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator or Competitor's bead-based activation reagent in ImmunoCult™-XF T Cell Expansion Medium supplemented with rhIL-2. Fold expansion was determined between 0 to 14 days. (Note that T cells were not reactivated during the course of expansion.)
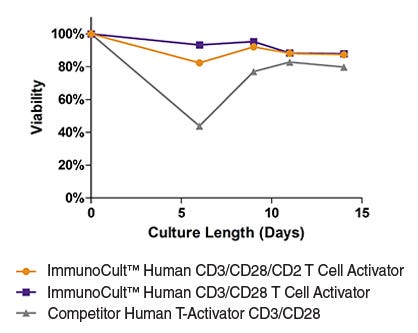
Figure 5. T cells stimulated with ImmunoCult™ Human T Cell Activators are highly viable.
T cells were expanded over 14 days with ImmunoCult™ Human CD3/CD28 T Cell Activator, ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator or Competitor's bead-based activation reagent in ImmunoCult™-XF T Cell Expansion Medium supplemented with rhIL-2. % viability was determined between 0 to 14 days. (Note that T cells were not reactivated during the course of expansion.)
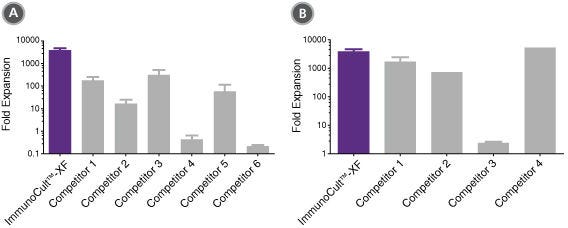
Figure 6. ImmunoCult™-XF T Cell Expansion Medium supports greater T cell expansion than other serum-free and serum-supplemented media.
T cells were activated with ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator, and cultured in (A) ImmunoCult™-XF T Cell Expansion Medium or serum-free competitor media with rhIL-2 in three replicate cultures per donor, or cultured in (B) ImmunoCult™-XF T Cell Expansion Medium or serum-supplemented competitor media with rhIL-2 in three replicate cultures per donor. T cells were stimulated with ImmunoCult™ Human CD3/CD28/CD2 T Cell Activator on day 0 and every 7 to 8 days for the duration of the culture. T cells were analyzed on day 21 for fold expansion relative to the initial cell seeding density.
(A) Compared to all serum-free competitor media tested, ImmunoCult™-XF T Cell Expansion Medium showed significantly higher expansion of total T cells. Competitors 1 to 6 represent serum-free competitor media. Each column with error bars represents the mean ± S.E.M. (p<5x10-13 for ImmunoCult™-XF T Cell Expansion Medium versus all other serum-free media, tested using the linear mixed effect model with linear regression, n = 4 to 19 donors).
(B) compared to all serum-supplemented competitor media tested, ImmunoCult™-XF T Cell Expansion Medium showed similar or significantly higher expansion of total T cells. Competitors 1 to 4 represent serum-supplemented competitor media. Each column with error bars represents the mean ± s.e.m. (p<0.0006 for ImmunoCult™-XF T Cell Expansion Medium versus all other serum-supplemented media except for competitor 4, tested using the linear mixed effect model with linear regression, n = 1 to 19 donors).
Try these isolation, activation and expansion reagents in your own lab.